| Date | May 2009 | Marks available | 4 | Reference code | 09M.2.hl.TZ1.8 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Explain and Sketch | Question number | 8 | Adapted from | N/A |
Question
The graph of the first ionization energy plotted against atomic number for the first twenty elements shows periodicity.
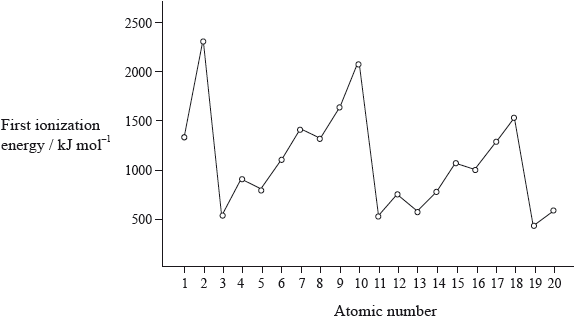
Explain how information from this graph provides evidence for the existence of main energy levels and sub-levels within atoms.
State what is meant by the term second ionization energy.
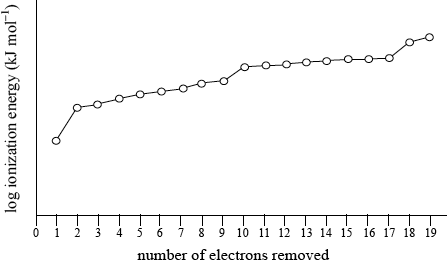
Sketch and explain the shape of the graph obtained for the successive ionization energies of potassium using a logarithmic scale for ionization energy on the y-axis against number of electrons removed on the x-axis.
State the full electronic configurations of copper, Cu, and the copper(I) ion, \({\text{C}}{{\text{u}}^ + }\).
Explain why copper(II) compounds in aqueous solution are coloured whereas scandium(III) compounds in aqueous solution are colourless.
Markscheme
(evidence for main levels)
highest values for noble gases / lowest values for alkali metals / OWTTE;
general increase across a period;
(evidence for sub-levels)
drop in I.E. from Be to B/Mg to Al/Group 2 to Group 3;
drop in I.E. from N to O/P to S/Group 5 to Group 6;
\({{\text{M}}^ + }{\text{(g)}} \to {{\text{M}}^{2 + }}{\text{(g)}} + {{\text{e}}^ - }\) / OWTTE ;
Accept e instead of e–.
Rough sketch to show:
Graph of successive ionization energies for potassium
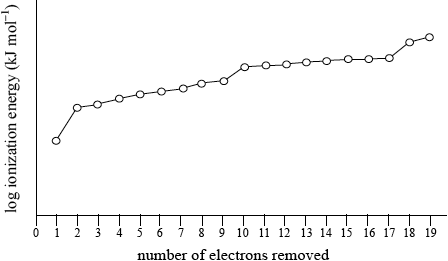
correct use of axes and one electron relatively easy to remove;
a jump in value then eight, another jump to another eight and finally another jump for the remaining two electrons ;
electronic configuration of \({\text{K}} = {\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}\) / first electron due to removal of \({\text{4}}{{\text{s}}^{\text{1}}}\), next eight due to third level/\({\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}\), next eight due to second level/\({\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}\) and last two due to removal of first level/\({\text{1}}{{\text{s}}^{\text{2}}}\);
the more electrons removed the more the positive nucleus attracts the remaining electrons and each main energy level is closer to the nucleus / OWTTE;
\({\text{(Cu) 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{3}}{{\text{d}}^{{\text{10}}}}/{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{1}}}\);
Do not accept [Ar]4s13d10.
\({\text{(C}}{{\text{u}}^ + }{\text{) 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}\);
Do not accept [Ar]3d10.
\({\text{C}}{{\text{u}}^{2 + }}\) has an incomplete d sub-level and \({\text{S}}{{\text{c}}^{3 + }}\) has no d electrons;
the d sub-level is split so the d electrons (in copper) can be excited by visible light / OWTTE;
Examiners report
Many candidates had difficulty correlating the graph of first ionization energy to main energy levels and sub-levels.
Commonly the graph looked very similar to the graph provided of first ionization energy against atomic number. Even the few candidates who seemed to understand the ideas involved with successive ionization energies drew only partial graphs and did not continue for the removal of all 19 electrons. Some teachers commented on the G2 forms that sketching the graph is beyond the scope of the course but it is clearly covered by AS 12.1.2.
In part (c), few candidates could correctly write the electron configurations of Cu and \({\text{C}}{{\text{u}}^ + }\), with many giving a full 4s orbital and only 9 electrons in the 3d orbitals. Candidates who managed to correctly write the electron configuration of Cu often removed a 3d electron when creating \({\text{C}}{{\text{u}}^ + }\).
Many candidates could explain why aqueous solutions of copper(II) compounds are coloured but those of scandium(III) compounds are not, but some candidates responded very weakly.

