| Date | May 2010 | Marks available | 4 | Reference code | 10M.2.sl.TZ2.5 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify and Outline | Question number | 5 | Adapted from | N/A |
Question
The periodic table shows the relationship between electron arrangement and the properties of elements and is a valuable tool for making predictions in chemistry.
The word redox comes from a combination of the terms reduction and oxidation. Redox reactions affect our daily lives.
The overall reaction that takes place in a voltaic cell is shown below.
\[{\text{Pb(s)}} + {\text{Pb}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}} \to {\text{2PbS}}{{\text{O}}_{\text{4}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Identify the property used to arrange the elements in the periodic table.
(ii) Outline two reasons why electronegativity increases across period 3 in the periodic table and one reason why noble gases are not assigned electronegativity values.
(i) Define the term first ionization energy of an atom.
(ii) Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
(iii) Explain why sodium conducts electricity but phosphorus does not.
(i) Determine the oxidation number of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\).
(ii) Deduce the oxidation and reduction half-equations taking place at the negative lead electrode (anode) and the positive lead(IV) oxide electrode (cathode). Deduce the oxidizing and reducing agents and state the direction of the electron flow between the electrodes.
(iii) In order to determine the position of three metals in a reactivity series, the metals were placed in different solutions of metal ions. The table below summarizes whether or not a reaction occurred.
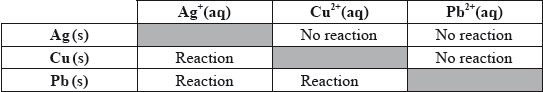
State the equations for the three reactions that take place. Use this information to place the metals Ag, Cu and Pb in a reactivity series, with the strongest reducing agent first, and explain your reasoning.
Markscheme
(i) atomic number / Z;
Accept nuclear charge / number of protons.
(ii) Across period 3:
increasing number of protons / atomic number / Z / nuclear charge;
(atomic) radius/size decreases / same shell/energy level / similar shielding/screening (from inner electrons);
No mark for shielding/screening or shielding/screening increases.
Noble gases:
do not form bonds (easily) / have a full/stable octet/shell/energy level / cannot attract more electrons;
Do not accept “inert” or “unreactive” without reference to limited ability/inability to form bonds or attract electrons.
Accept the following as alternative to M3.
no attraction for electrons/full outer shell / stable/inert/do not form bonds (readily/easily);
(i) energy/enthalpy change/required/needed to remove/knock out an electron (to form +1/uni-positive/\({{\text{M}}^{ + 1}}\) ion);
in the gaseous state;
Award [1] for M(g) \( \to \) M+(g) + e–.
Award [2] for M(g) \( \to \) M+(g) + e– with reference to energy/enthalpy change.
(ii) increasing number of protons/atomic number/Z/nuclear charge;
radius/size decreases / same shell/energy level / similar shielding/screening (from inner electrons);
No mark for shielding/screening or shielding/screening increases.
(iii) Na: delocalized electrons / mobile sea of electrons / sea of electrons free to move;
No mark for just “mobile electrons”.
(i) \({\text{Pb: 0, Pb}}{{\text{O}}_{\text{2}}}{\text{: }} + 4{\text{, PbS}}{{\text{O}}_{\text{4}}}{\text{: }} + 2\);
Need sign for mark.
Do not accept notations such as 4+, 2+ or IV, II.
(ii) Negative/–/anode
\({\text{Pb(s)}} + {\text{SO}}_4^{2 - }{\text{(aq)}} \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{e}}^ - }/{\text{Pb(s)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Positive/+/cathode
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}/\)
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}/\)
\({\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{PbS}}{{\text{O}}_4}{\text{(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\);
Accept Pb4+ + 2e– \( \to \) Pb2+.
Ignore state symbols.
Allow e instead of e–.
oxidizing agent is \({\text{Pb}}{{\text{O}}_{\text{2}}}\)/lead(IV) oxide/lead dioxide and reducing agent is Pb/lead;
from negative/–/anode/Pb to positive/+/cathode/\({\text{Pb}}{{\text{O}}_{\text{2}}}\) (through the external circuit/wire);
(iii) \({\text{Pb(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\)
\({\text{Pb(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}\)
\({\text{Cu(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}\)
Award [2] for three correct, award [1] for any two correct, one correct scores no mark.
Ignore state symbols.
Penalize unbalanced equations once only.
Pb is a stronger reducing agent than Cu and/or Ag / Pb most reactive as it can reduce/displace both \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{A}}{{\text{g}}^ + }\);
Cu is a stronger reducing agent than Ag but not Pb / Cu in the middle (of the three) as it can reduce/displace \({\text{A}}{{\text{g}}^ + }\) but not \({\text{P}}{{\text{b}}^{2 + }}\);
Accept converse argument.
Decreasing order: Pb, Cu, Ag / \({\text{Pb}} > {\text{Cu}} > {\text{Ag}}\);
Do not accept: Pb2+, Cu2+, Ag+.
Examiners report
Parts (a) and (b) were reasonably well answered. Generally the understanding of electronegativity was good, but some made the error of stating that it was the attraction of one electron only; others did not clarify that it is the ability of an atom to attract a shared electron pair in a covalent bond. The reason for the increase in electronegativity across period 3 was sometimes incomplete with candidates not mentioning both the increase in number of protons and the decrease of atomic size.
Although the definition of first ionisation energy was generally well known quite a few candidates only gave one reason for the increase across a period and some referred to the number of electrons in the outer shell as a reason for the general increase. Many candidates did not refer to delocalized electrons when explaining the difference in electrical conductivity between sodium and phosphorus.
The oxidation numbers of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\) were given correctly by many candidates; however, incorrect responses included 0, II and IV or as 4+ and 2+.
A common mistake was to give +10 for the oxidation state for lead in lead(IV) sulfate presumably as candidates mistakenly assigned an oxidation number of –2 to sulfur. The half reactions for the lead-acid storage battery proved to be difficult with few being able to deduce the half-equation at the cathode, although \({\text{P}}{{\text{b}}^{4 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }}\) was accepted. The number of candidates providing the more accurate version of the reaction at the cathode, namely: \({\text{Pb}}{{\text{O}}_{\text{2}}} + {\text{4}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{P}}{{\text{b}}^{2 + }} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}\) was very small. In addition, many candidates interchanged the oxidizing and reducing agents. Most candidates were able to place Ag, Cu and Pb in the correct order of reactivity although equations were often unbalanced or included incorrect ionic charges with species such as \({\text{A}}{{\text{g}}^{2 + }}\) or \({\text{C}}{{\text{u}}^ + }\). Many could not explain the position of metals in the reactivity series clearly with statements such as “Pb is the strongest reducing agent because it reduces Cu and Ag” instead of \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{A}}{{\text{g}}^ + }\).

