| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.hl.TZ1.7 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce | Question number | 7 | Adapted from | N/A |
Question
Bromine is a member of group 7, the halogens.
Iron is a transition metal.
Freshly prepared iron(II) bromide can be electrolysed both in the liquid state and in aqueous solution.
Explain the trend in reactivity of the halogens.
Deduce, using equations where appropriate, if bromine reacts with sodium chloride solution and with sodium iodide solution.
Describe the bonding in metals and explain their malleability.
List three characteristic properties of transition elements.
Identify the type of bonding between iron and cyanide in \({{\text{[Fe(CN}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 - }}\).
Deduce the oxidation number of iron in \({{\text{[Fe(CN}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 - }}\).
Draw the abbreviated orbital diagram for an iron atom using the arrow-in-box notation to represent electrons.
Draw the abbreviated orbital diagram for the iron ion in [Fe(CN)6]3– using the arrow-in-box notation to represent electrons.
Describe, using a diagram, the essential components of an electrolytic cell.
Describe the two ways in which current is conducted in an electrolytic cell.
Predict and explain the products of electrolysis of a dilute iron(II) bromide solution.
Identify another product that is formed if the solution of iron(II) bromide is concentrated.
Explain why this other product is formed.
Markscheme
reactivity decreases down group;
as atomic radius increases / more electron shells;
attraction of nucleus on electrons decreases / electron affinity decreases;
Accept opposite argument for “up the group”.
no reaction with NaCl;
\({\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2NaI(aq)}} \to {\text{2NaBr(aq)}} + {{\text{I}}_2}{\text{(aq)}}\);
Accept ionic equation.
Ignore state symbols.
(electrostatic attraction between a) lattice of positive ions/cations and delocalized/sea of electrons;
Accept suitable diagram.
atoms/ions/layers (of positive ions) can slide over each other / OWTTE;
without change in the bonding forces / OWTTE;
variable oxidation numbers/valency
form complex (ions)
form coloured compounds/ions
catalytic (behaviour)
Award [2] for any three, [1] for any two.
dative (covalent)/coordinate;
III / \( + 3\);
Penalize incorrect format such as 3+ only if not penalized in 4 (b).
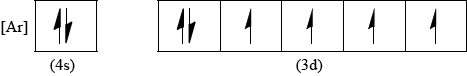
Penalise missing [Ar] only once in (v) and (vi).
Do not accept full orbital diagram; penalise only once in (v) and (vi).
Accept full or half-arrows in (v) and (vi).
Ignore absence of labels 4s and 3d.
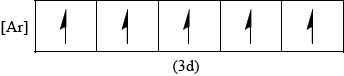 ;
;
Accept empty 4s box in (vi).
No ECF from (iv).
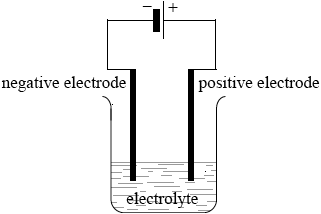
clear diagram containing all elements (power supply, connecting wires,
electrodes, container and electrolyte);
Accept power supply if shown as conventional long/short lines (as in diagram above) or clearly labelled DC power supply.
labelled positive electrode/anode and negative electrode/cathode;
Accept positive and negative by correct symbols near power supply.
labelled electrolyte/FeBr2(l)/FeBr2(aq);
State must be included for FeBr2.
Electrolyte: positive ions/cations move to negative electrode/cathode and negative ions/anions to positive electrode/anode;
Conductors: electrons flow from negative pole of battery to positive pole of battery / OWTTE;
Look at diagram in (i) for possible clarification of electron flow.
Award [1 max] for “electrons in wire/external circuit and ions in solution”.
Negative electrode/cathode:
\({{\text{H}}_{\text{2}}}\);
\({E^\Theta }{\text{(}}{{\text{H}}_{\text{2}}}{\text{)}}\) is less negative than \({E^\Theta }{\text{(Fe)}}\) / Fe is more reactive than \({{\text{H}}_{\text{2}}}\) / \({{\text{H}}_{\text{2}}}\) is lower in reactivity series / \({{\text{H}}^ + }\) more easily reduced than Fe2+ / OWTTE;
Positive electrode/anode:
\({{\text{O}}_{\text{2}}}\);
\({E^\Theta }{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}\) is less positive than \({E^\Theta }{\text{(B}}{{\text{r}}_{\text{2}}}{\text{)}}\) / in a dilute \({\text{B}}{{\text{r}}^ - }\) solution \({\text{O}}{{\text{H}}^ - }{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}\) is
preferably discharged / OWTTE;
Award [3 max] if electrodes reversed or omitted.
\({\text{B}}{{\text{r}}_{\text{2}}}\);
Accept Fe.
\({\text{2B}}{{\text{r}}^ - } \rightleftharpoons {\text{B}}{{\text{r}}_2} + {\text{2}}{{\text{e}}^ - }\) shifts to the right;
Accept similar reason for Fe.
Examiners report
This was the least popular of the Section B questions. In (a) (i) the trend was generally correctly identified but the reasons were not clear, many confusing electronegativity with electron affinity. Most knew about the reactions (or lack thereof) of bromine but the equations were sometimes unbalanced or included halogen atoms rather than molecules.
This was the least popular of the Section B questions. In (a) (i) the trend was generally correctly identified but the reasons were not clear, many confusing electronegativity with electron affinity. Most knew about the reactions (or lack thereof) of bromine but the equations were sometimes unbalanced or included halogen atoms rather than molecules.
There was a tendency to describe the bonding of metals in terms of nuclei rather than cations and malleability was not well understood.
The properties in (b) (ii) were surprisingly poor. Many suggested that the metals themselves are coloured rather than the compounds, for instance.
The bonding in (iii) was not well known but the oxidation number was generally answered correctly.
In (v), some candidates gave the full orbital diagram, some omitted [Ar] – and some just got it wrong!
The diagrams in (c) were poorly presented and often inaccurate (much confusion with a voltaic cell) and there was little understanding of how current was transmitted.
The diagrams in (c) were poorly presented and often inaccurate (much confusion with a voltaic cell) and there was little understanding of how current was transmitted.
In (iii), few candidates correctly predicted the products of electrolysis of dilute iron bromide, with many seeming to ignore the presence of hydrogen ions/hydroxide ions/water; correct explanations in terms of electrode potentials or preferential discharge were rare.
In (iii), few candidates correctly predicted the products of electrolysis of dilute iron bromide, with many seeming to ignore the presence of hydrogen ions/hydroxide ions/water; correct explanations in terms of electrode potentials or preferential discharge were rare. Despite this, bromine was often correctly identified in (iv).
In (v), few understood the impact of concentrating the electrolyte.

