| Date | May 2015 | Marks available | 1 | Reference code | 15M.3.hl.TZ1.2 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Deduce | Question number | 2 | Adapted from | N/A |
Question
The structure of an unknown compound A with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}\) can be determined using information from a variety of analytical techniques.
The infrared (IR) spectrum of A is shown below.
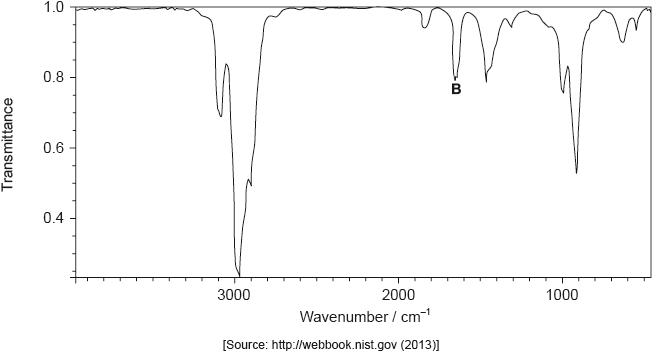
The mass spectrum of A is shown below.
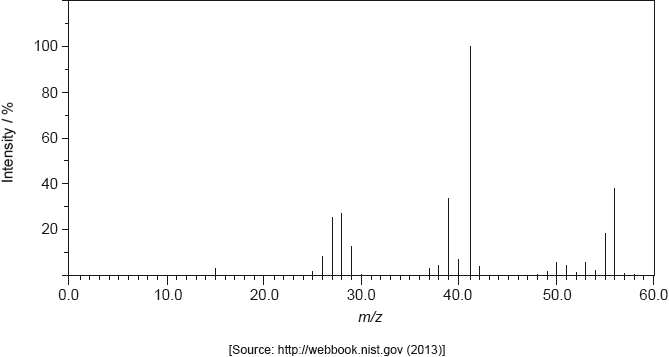
Deduce the formula of the molecular ion from the mass spectrum.
Explain the presence of a doublet in the high-resolution proton nuclear magnetic resonance (1H NMR) spectrum of A.
One isomer of A has only one signal in its \(^{\text{1}}{\text{H}}\) NMR spectrum. Deduce the structural formula of this isomer.
Markscheme
\({{\text{C}}_4}{\text{H}}_8^ + \);
Penalize missing charge only once in (i) and (ii).
produced by \({\text{(Hs in)}} = {\text{C}}{{\text{H}}_2}\) group;
adjacent C has 1 H atom;
\(n + 1\);
due to relative/(two) different orientations/alignment of spin of nuclei/protons/hydrogens (with applied/external magnetic field);
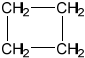 ;
;
Accept full or condensed structural formula.
Examiners report
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.
This question focused on some of the fundamental spectroscopic techniques (MS, IR and \(^{\text{1}}{\text{H}}\) NMR) used in analytical chemistry. The better candidates did well on this question though few scored full marks. In (a), the most common mistake was omission of the positive charge in (i). One G2 comment stated that isotopic effects in mass spectra with regard to the determination of the molecular ion peak would confuse students. This generally was not the case and although most got the \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\) formula a large majority of candidates simply did not read the question correctly which specifically asked for the formula of the molecular ion. In (ii) the correct formulas of the fragments were usually given. In (b), C=C was usually cited as the correct structural formula in (ii). The weaker candidates struggled with explaining the doublet in (iii). Cyclobutane was obtained by a large number of candidates in (iv). In (c) (i), the better candidates scored all three marks. In (ii), an understanding of the fingerprint region was poorly conveyed. There were two parts to this question – an outline of what happens on a molecular level when radiation in the fingerprint region is absorbed and how this region is used in chemical analysis. One G2 comment referred to the fact that the fingerprint region is not explicitly mentioned in the guide. Although this is true per se AS 3.2 does require a description of how the information from an IR spectrum can be used to identify bonds, and it would be assumed that the fingerprint region would be discussed in the context of teaching IR spectroscopy as part of the IB chemistry programme.

