| Date | November 2013 | Marks available | 3 | Reference code | 13N.3.hl.TZ0.2 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | 2 | Adapted from | N/A |
Question
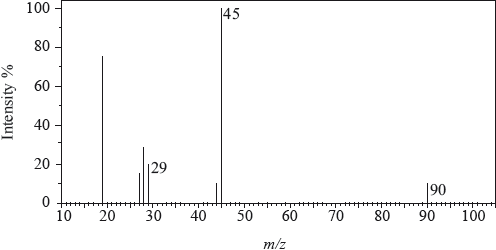
The mass spectrum of an unknown acidic compound, X, with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\), is shown below.
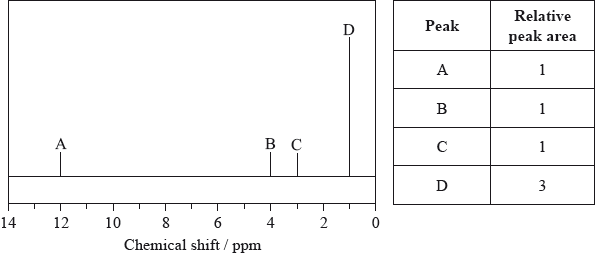
The low-resolution \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows four peaks. A simplified representation is shown alongside a table with relative peak areas.
Determine the relative molecular mass, to the nearest integer, of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formula of the fragment responsible for the peak at 45.
Deduce the formula of the fragment responsible for the peak at 29.
Identify the group responsible for the peak at D.
Suggest a possible structure for X.
Peak B shows the following splitting pattern in the high-resolution spectrum.
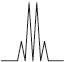
Explain the splitting pattern, indicating the hydrogen responsible for peak B.
Markscheme
90;
\({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_3^ + \);
Penalize missing positive charge of ion only once in (a).
\({\text{COO}}{{\text{H}}^ + }\);
Accept C2H5O+.
Penalize missing positive charge of ion only once in (a).
\({\text{CH}}{{\text{O}}^ + }/{\text{CO}}{{\text{H}}^ + }{\text{ }}\);
Accept C2H5+/CH3CH2+.
Penalize missing positive charge of ion only once in (a).
\({\text{C}}{{\text{H}}_3}\)/methyl;
\({\text{C}}{{\text{H}}_3}{\text{CH(OH)COOH}}\);
Allow full or condensed structural formula.
quartet means next C has 3 H atoms / is \({\text{C}}{{\text{H}}_3}\);
due to the CH group;
due to relative orientation of spinning nuclei/protons;
with relative probabilities of 1,3,3,1;
OH group results in no splitting (due to rapid proton exchange);
Examiners report
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.

