| Date | May 2013 | Marks available | 1 | Reference code | 13M.3.hl.TZ2.A4 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Describe | Question number | A4 | Adapted from | N/A |
Question
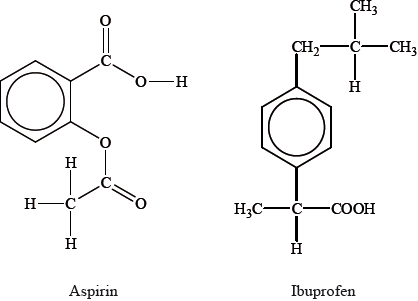
Another painkiller is aspirin. The structures of aspirin and ibuprofen are:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of one of the intermediate compounds formed during the synthesis of the painkiller ibuprofen is shown below. The peaks labelled A to G are not fully expanded to show the splitting but the integration trace for each peak is included.
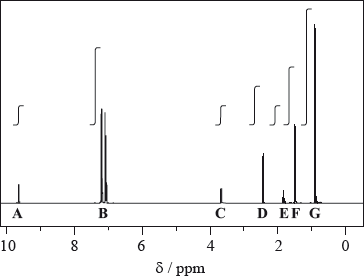
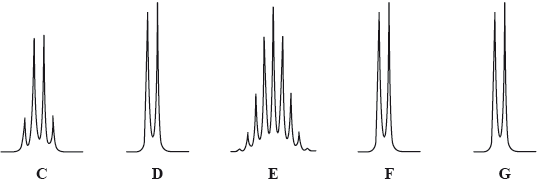
The peak labelled A is a singlet. The two peaks labelled B centred at 7.1 ppm are due to the four hydrogen atoms on the benzene ring. The expansions to show the splitting for the other five peaks are shown below.
The structure of the intermediate compound is given below, with seven of the hydrogen atoms labelled.
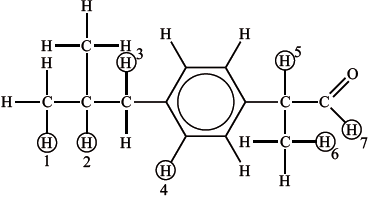
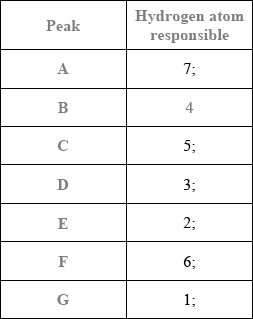
Deduce which labelled hydrogen atoms are responsible (wholly or in part) for each of the peaks and complete the table.
State the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of aspirin (ignore the peaks due to the hydrogen atoms on the benzene ring and the reference sample).
Describe the splitting pattern for each of the peaks given in (b) (i).
State how the infrared spectra of aspirin and ibuprofen will differ in the region 1700–1750 cm–1.
Markscheme
two/2;
both are singlets / OWTTE;
aspirin will have two (sharp) peaks / wider/greater absorption (due to two C=O groups);
ibuprofen will have one (sharp) peak / narrower/less absorption (due to one C=O group);
Examiners report
Overall, there was a mixed response to part a) of this question with some students scoring full marks
and others only achieving 1 or 2 marks.
Parts (b) (i) and (ii) showed many correct answers although in part (iii) ,candidates often referred to number of carbonyl groups in each of the two painkillers but established no connection with how this would affect the IR spectrum of each compound.
Parts (b) (i) and (ii) showed many correct answers although in part (iii) ,candidates often referred to number of carbonyl groups in each of the two painkillers but established no connection with how this would affect the IR spectrum of each compound.
Parts (b) (i) and (ii) showed many correct answers although in part (iii) ,candidates often referred to number of carbonyl groups in each of the two painkillers but established no connection with how this would affect the IR spectrum of each compound.

