| Date | November 2012 | Marks available | 1 | Reference code | 12N.3.hl.TZ0.A3 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Deduce | Question number | A3 | Adapted from | N/A |
Question
The molecule of an unknown straight-chain compound consists of 4 carbon, 8 hydrogen, and 1 oxygen atoms. The 1H NMR spectrum of the compound is given below (the numbers next to integration traces correspond to areas under each peak).
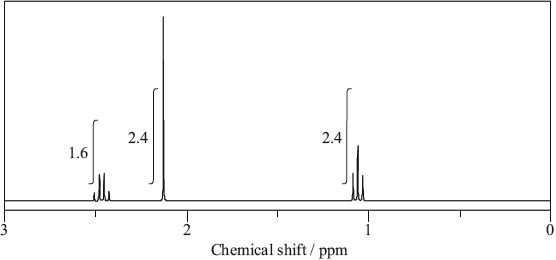
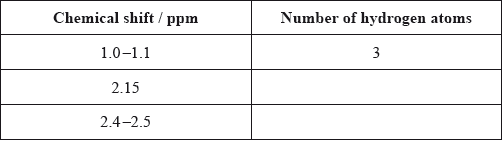
Calculate the number of hydrogen atoms for peaks with chemical shifts of 2.15 and 2.4–2.5 ppm. An example for the peak at 1.0–1.1 ppm is given.
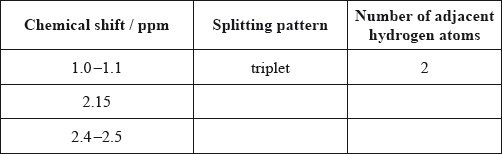
Analyse the splitting pattern of each peak and determine the relative positions of hydrogen atoms in the molecule. One example is given.
Using the information from (a) and (b), deduce the structural formula of the organic compound.
Markscheme
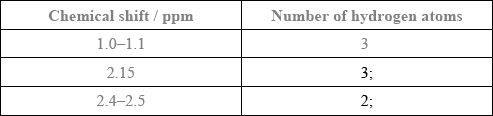
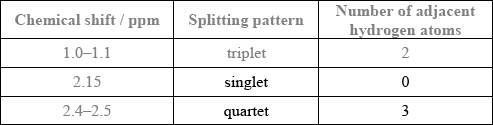
Award [1] for both splitting patterns correct.
Award [1] for both number of adjacent hydrogen atoms correct.
\({\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\);
Accept more detailed formula.
Examiners report
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.
Option A proved to be very popular. Some candidates had difficulty explaining the purpose of the monochromator and some muddled Qualitative and Quantitative, but a reasonable proportion explained the latter. Many students were able to describe the practical method of column chromatography but were not able to explain the process in terms of adsorption, partition and retention. While many candidates knew about ‘d’ orbital splitting some forgot to explain the change in magnitude of the splitting, and a significant few thought that fewer ‘d’ electrons in the \({\text{C}}{{\text{r}}^{3 + }}\) ion would cause less repulsion and so less splitting.

