| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.sl.TZ2.7 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Define | Question number | 7 | Adapted from | N/A |
Question
Some reactions of but-2-ene are given below.
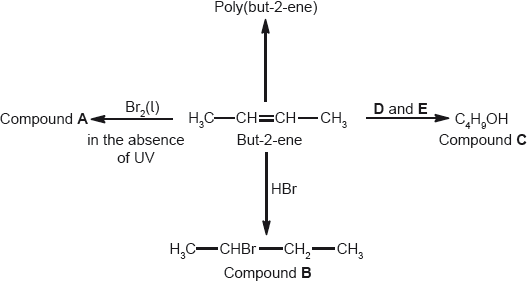
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
State the names of the reagents D and E.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed directly from compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\).
(i) State the reagent and the conditions required for this reaction.
(ii) State the name of the type of reaction occurring in this conversion.
Compound C can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
Define the term average bond enthalpy.
Deduce the equation for the complete combustion of compound C.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the complete combustion of compound C when all reactants and products are in the gaseous state, using table 10 of the data booklet.
Markscheme
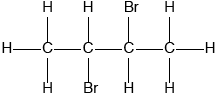 ;
;
Accept bromine atoms cis to each other.
2,3-dibromobutane;
Do not penalize the incorrect use of spaces, comma or hyphen.
red/brown/orange/yellow to colourless/decolourized;
Do not accept clear.
Do not accept just “decolourized”.
water;
sulfuric acid / phosphoric acid;
Accept formulas instead of names.
(i) (synthesis of) plastics/polymers/organic materials not naturally available / synthetic materials;
wide range of uses/physical properties / versatile;
large industry / many tons of plastics consumed by society / OWTTE;
Do not accept “useful” for M2.
Award [1 max] if specific addition polymer and its use is given.
Penalize reference to condensation polymers once only.
(ii) 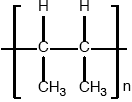 ;
;
Ignore n.
Brackets are not required for the mark, but continuation bonds are.
Do not penalize if methyl groups are trans to each other.
(i) aqueous sodium hydroxide/NaOH/potassium hydroxide/KOH and warm/heat/reflux;
(ii) (nucleophilic) substitution;
Accept (nucleophilic) displacement.
(i) carbonyl;
Accept ketone.
(ii) 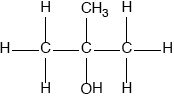 ;
;
Accept condensed or full structural formula.
hydrogen bonding in compound C;
dipole-dipole forces in C / C is more polar;
C has greater molar mass/more dispersion/London/instantaneous induced dipole-induced dipole forces/van der Waal forces;
Accept converse argument.
Award [1 max] for stronger intermolecular forces.
energy required to break (1 mol of) a (covalent) bond in a gaseous molecule/state;
Accept energy released when (1 mol of) a (covalent) bond is formed in a gaseous molecule/state / energy change when (1 mol of) bonds are formed or broken in the gaseous molecule/state.
average value in similar compounds / OWTTE;
\({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{6}}{{\text{O}}_2}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{5}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\);
Ignore state symbols.
Bonds broken:
3C–C + 9C–H + 1C–O + 1O–H + 6O=O /
\(3 \times 347 + 9 \times 413 + 1 \times 358 + 1 \times 464 + 6 \times 498/8568{\text{ (kJ)}}\);
Bonds formed:
8C=O + 10O–H / \(8 \times 746 + 10 \times 464/10608{\text{ (kJ)}}\);
\(\Delta H = (8568 - 10608) = - 2040{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +2040 (kJ mol–1).
Examiners report
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.
The few who opted for this option, showed a good knowledge. The drawing of structural formulae and naming was good. The reagent and conditions for the reaction was less well recalled. In 7ci, most students scored at least one mark, but lost the second. There was a lack of awareness of the importance of the system being aqueous in the conversion to the alcohol and a fully correct answer was very rare, as was the identification of the functional group. In the volatility question, most were aware of hydrogen bonding, but the fact that C also has greater other forces due to its greater mass was not present in most answers. The gaseous mark was often present, but the averaging over a range of compounds was not. With the calculation of enthalpy quite a few candidates benefitted from transferred error, from an incorrect equation.

