| Date | November 2011 | Marks available | 1 | Reference code | 11N.1.sl.TZ0.14 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Deduce | Question number | 14 | Adapted from | N/A |
Question
A student measured the temperature of a reaction mixture over time using a temperature probe. By considering the graph, which of the following deductions can be made?
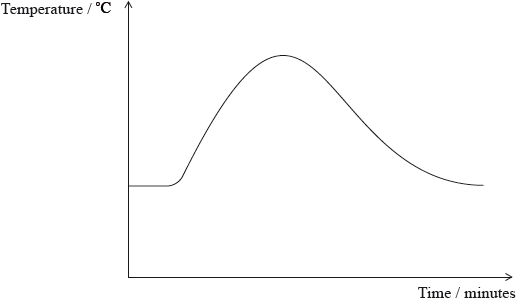
I. The reaction is exothermic.
II. The products are more stable than the reactants.
III. The reactant bonds are stronger than the product bonds.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
There were two G2 comments on this question, both suggesting that the graph given was confusing to candidates. In this question candidates had to use a combination of ideas to ascertain that the correct answer is A, namely I. and II. From the graph shown, candidates need to realise that the reaction is exothermic, and therefore from this information, the products are more stable than the reactants. 55% of candidates got the correct answer.

