| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.hl.TZ2.30 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | Derive | Question number | 30 | Adapted from | N/A |
Question
Two cells undergoing electrolysis are connected in series.
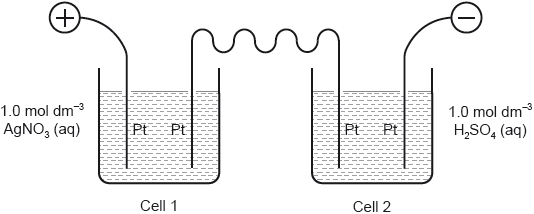
If \(x\) g of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
Ar(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
A. \(\frac{x}{{108}} \times \frac{1}{4} \times 22.7\)
B. \(\frac{x}{{108}} \times 4 \times 22.7\)
C. \(\frac{x}{{108}} \times \frac{1}{2} \times 22.7\)
D. \(\frac{x}{{108}} \times 2 \times 22.7\)
Markscheme
A
Examiners report
[N/A]
Syllabus sections
Show 132 related questions
- 18M.2.hl.TZ2.4c: Calculate the standard electrode potential, in V, for the BrO3−/Br− reduction half‑equation...
- 18M.2.hl.TZ2.4b: The change in the free energy for the reaction under standard conditions, ΔGΘ, is −514 kJ at...
- 18M.2.hl.TZ2.3c.v: Deduce the gas formed at the anode (positive electrode) when graphite is used in place of...
- 18M.2.hl.TZ1.6e: Determine the loss in mass of one electrode if the mass of the other electrode increases by...
- 18M.2.hl.TZ1.6d: Calculate the cell potential, in V, using section 24 of the data booklet.
- 18M.1.hl.TZ2.31: What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?
- 18M.1.hl.TZ1.31: What does not affect the mass of products formed in electrolysis of an aqueous solution? A. ...
- 18M.1.hl.TZ1.30: Which combination would electroplate an object with copper?
- 17N.2.hl.TZ0.7e: State and explain the products of electrolysis of a concentrated aqueous solution of sodium...
- 17N.2.hl.TZ0.7c: Calculate the cell potential, in V, when the standard iodine and manganese half-cells are...
- 17N.2.hl.TZ0.7b: Predict, giving a reason, the direction of movement of electrons when the standard nickel and...
- 17N.1.hl.TZ0.31: What are the products when an aqueous solution of copper(II) sulfate is electrolysed using...
- 17M.2.hl.TZ2.2c: Zinc is used to galvanize iron pipes, forming a protective coating. Outline how this process...
- 17M.2.hl.TZ2.2b.ii: Calculate the Gibbs free energy, ΔG θ, in kJ, which is released by the corrosion of 1 mole of...
- 17M.2.hl.TZ2.2b.i: Corrosion of iron is similar to the processes that occur in a voltaic cell. The initial steps...
- 17M.1.hl.TZ2.31: What are the relative volumes of gas given off at E and F during electrolysis of the two...
- 17M.1.hl.TZ2.30: What is the standard half-cell potential of copper if the “zero potential reference...
- 17M.2.hl.TZ1.3c.ii: Comment on the spontaneity of this reaction by calculating a value for \(\Delta {G^\theta...
- 17M.2.hl.TZ1.3b.ii: Identify, from the table, a non-vanadium species that could convert...
- 17M.2.hl.TZ1.3b.i: Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no...
- 17M.1.hl.TZ1.31: In the electrolysis of aqueous potassium nitrate, KNO3(aq), using inert electrodes, 0.1 mol...
- 17M.1.hl.TZ1.30: Which statement is correct for the overall reaction in a voltaic cell? 2AgNO3(aq) + Ni(s) →...
- 16N.3.hl.TZ0.21b: A concentration cell is an example of an electrochemical cell. (i) State the difference...
- 16N.2.hl.TZ0.4k: A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell,...
- 16N.2.hl.TZ0.4j: Standard electrode potentials are measured relative to the standard hydrogen electrode....
- 16N.2.hl.TZ0.4i: Magnesium chloride can be electrolysed. (i) Deduce the half-equations for the reactions at...
- 16N.1.hl.TZ0.33: An iron rod is electroplated with silver. Which is a correct condition for this process? A....
- 16N.1.hl.TZ0.32: Which signs for both Eθcell and ΔGθ result in a spontaneous redox reaction occurring under...
- 16M.2.hl.TZ0.4b: Tin can also exist in the +4 oxidation state. Vanadium can be reduced from an oxidation...
- 16M.1.hl.TZ0.33: z...
- 16M.1.hl.TZ0.32: Which...
- 15M.1.hl.TZ1.31: Two half-cells are connected via a salt bridge to make a voltaic cell. Which statement about...
- 15M.1.hl.TZ1.32: Which signs are correct for a spontaneous redox reaction?
- 15M.1.hl.TZ1.33: Consider the standard electrode...
- 15M.1.hl.TZ2.32: The standard electrode potentials for three reactions involving copper and copper ions...
- 15M.1.hl.TZ2.33: The same quantity of electricity is passed through separate dilute aqueous solutions of...
- 15M.2.hl.TZ1.6f.i: Define the term standard electrode potential, \({E^\Theta }\).
- 15M.2.hl.TZ1.6d.ii: Predict the products formed at the electrodes during the electrolysis of concentrated aqueous...
- 15M.2.hl.TZ1.6f.ii: Draw a labelled diagram for the voltaic cell in which the following reaction...
- 15M.2.hl.TZ2.8e.i: Define the term standard electrode potential.
- 15M.2.hl.TZ2.8e.iii: Predict the balanced equation for the spontaneous reaction which will produce a current in...
- 15M.2.hl.TZ2.8e.iv: Identify the negative and the positive electrodes in this cell.
- 15M.2.hl.TZ2.8e.v: Predict the direction of movement of electrons in the external circuit.
- 15M.2.hl.TZ2.8e.vi: State the directions in which the negative ions (anions) and the positive ions (cations) flow...
- 14M.1.hl.TZ1.33: The overall equation of a voltaic cell...
- 14M.1.hl.TZ2.34: What is the cell potential, in V, of the reaction...
- 14M.1.hl.TZ2.33: Which components are used to make the standard hydrogen electrode? A. ...
- 14M.2.hl.TZ1.6b: (i) Deduce the order of reactivity of these four metals, from the least to the most...
- 14M.2.hl.TZ1.6c: (i) State the half-equation for the reaction that occurs at each electrode. Positive...
- 14M.2.hl.TZ1.6d: (i) Determine the mass of copper produced at one of the electrodes in cell 2 if the tin...
- 14M.2.hl.TZ2.8g: (i) Draw a labelled diagram of a suitable apparatus for the electrolysis. (ii) ...
- 14N.1.hl.TZ0.32: Consider the following standard electrode potentials. ...
- 14N.1.hl.TZ0.33: A number of molten metal chlorides are electrolysed, using the same current for the same...
- 14N.2.hl.TZ0.10f: (i) An aqueous solution of sodium chloride is electrolysed using inert electrodes....
- 14N.2.hl.TZ0.10g: Describe how electrolysis can be used to electroplate a bracelet with a layer of silver...
- 13N.1.hl.TZ0.33: Consider the following two standard electrode potentials at 298 K. ...
- 13N.1.hl.TZ0.34: What happens during the electrolysis of concentrated aqueous potassium chloride? I. ...
- 13N.2.hl.TZ0.6a: Define the term standard electrode potential, \({E^\Theta }\).
- 13N.2.hl.TZ0.6c: In the two experiments below, predict whether a reaction would occur and deduce an equation...
- 13N.2.hl.TZ0.6d.i: Using Table 14 of the Data Booklet, state the balanced half-equation for the reaction that...
- 13N.2.hl.TZ0.6d.ii: Calculate the cell potential in V.
- 13N.2.hl.TZ0.6d.iii: On the diagram above label with an arrow • the direction of electron flow in the wire • the...
- 13M.1.hl.TZ1.33: The standard electrode potentials of some half-reactions are given below. ...
- 13M.1.hl.TZ1.31: An aqueous solution of a metal salt is electrolysed. Which factor will have no effect on the...
- 13M.2.hl.TZ1.7c.iv: Identify another product that is formed if the solution of iron(II) bromide is concentrated.
- 13M.2.hl.TZ1.4f: Predict the sign of \(\Delta {G^\Theta }\) for this reaction.
- 13M.2.hl.TZ1.7c.iii: Predict and explain the products of electrolysis of a dilute iron(II) bromide solution.
- 13M.2.hl.TZ1.4e.ii: Explain the sign of the calculated standard electrode potential.
- 13M.2.hl.TZ1.7c.v: Explain why this other product is formed.
- 13M.1.hl.TZ2.32: Which statement is correct for electroplating an object with gold? A. The object must be...
- 13M.2.hl.TZ2.7d.ii: Using Table 14 of the Data Booklet, calculate the cell potential,...
- 13M.2.hl.TZ2.7e.iii: State why copper electrodes cannot be used in the electrolysis of water. Suggest instead...
- 13M.2.hl.TZ2.7c.i: Describe the standard hydrogen electrode including a fully labelled diagram.
- 13M.2.hl.TZ2.7e.iv: Deduce the half-equations for the reactions occurring at the positive electrode (anode) and...
- 13M.2.hl.TZ2.7c.ii: Define the term standard electrode potential, \({E^\Theta }\).
- 13M.2.hl.TZ2.7e.i: Deduce the sign of the standard free energy change, \(\Delta {G^\Theta }\), for any...
- 13M.2.hl.TZ2.7e.ii: State why dilute sulfuric acid needs to be added in order for the current to flow in the...
- 13M.2.hl.TZ2.7e.v: Deduce the overall cell reaction, including state symbols.
- 13M.2.hl.TZ2.7e.vii: Comment on what is observed at both electrodes.
- 13M.2.hl.TZ2.7f: Two electrolytic cells are connected in series (the same current passes through each cell)....
- 13M.1.sl.TZ2.25: The overall reaction in the voltaic cell below is: Which statement is correct for the...
- 12N.2.hl.TZ0.4b: (i) Draw an annotated diagram of the electrolytic cell in process 1 and identify the...
- 10N.1.hl.TZ0.32: A voltaic cell is made by connecting two half-cells represented by the half-equations...
- 10N.1.hl.TZ0.33: For the electrolysis of aqueous copper(II) sulfate, which of the following statements is...
- 10N.2.hl.TZ0.4b: (i) Explain how molten magnesium chloride conducts an electric current. (ii) ...
- 09N.1.hl.TZ0.31: Which are necessary conditions for the standard hydrogen electrode to have an \({E^\Theta }\)...
- 09N.2.hl.TZ0.4b.i: Deduce a balanced equation for the overall reaction which will occur spontaneously when these...
- 09N.2.hl.TZ0.4b.ii: Determine the cell potential when the two half-cells are connected.
- 10M.2.hl.TZ1.6d.iii: Two electrolytic cells are connected in series as shown in the diagram below. In one there is...
- 10M.2.hl.TZ1.6a.iii: Using Table 14 of the Data Booklet, calculate the cell potential for this cell.
- 10M.2.hl.TZ1.6b: The standard electrode potentials for three other electrode systems are given below. (i) ...
- 10M.2.hl.TZ1.6c: These values were obtained using a standard hydrogen electrode. Describe the materials and...
- 10M.3.hl.TZ1.C3: Discuss the production of chlorine and sodium hydroxide from brine using a membrane cell....
- 10M.2.hl.TZ2.8b: (i) Molten sodium chloride is electrolysed in a cell using inert electrodes. State the...
- 10M.2.hl.TZ2.8c: Electroplating is an important application of electrolytic cells with commercial...
- 09M.1.hl.TZ1.32: Consider these standard electrode...
- 09M.2.hl.TZ1.7a.i: Define the term standard electrode potential and state the meaning of the minus sign in the...
- 09M.2.hl.TZ1.7a.ii: Calculate the value for the standard electrode potential for the cobalt half-cell.
- 09M.2.hl.TZ1.7c.i: Draw a labelled diagram of the cell. Use an arrow to show the direction of the electron flow...
- 09M.2.hl.TZ1.7c.ii: Give the formulas of all the ions present in the solution.
- 09M.2.hl.TZ1.7c.iv: Deduce the molar ratios of the products obtained at the two electrodes.
- 09M.2.hl.TZ1.7d.i: concentrated sodium chloride
- 09M.2.hl.TZ1.7a.v: Explain the function of the salt bridge in an electrochemical cell.
- 09M.2.hl.TZ1.7a.iv: Deduce the equation for the spontaneous reaction taking place when the iron half-cell is...
- 09M.2.hl.TZ1.7c.iii: Predict the products obtained at each electrode and state the half-equation for the formation...
- 09M.1.hl.TZ2.32: What is the cell potential, in V, for the reaction that occurs when the following two...
- 09M.2.hl.TZ2.6c.v: Electroplating is an important application of electrolysis. State the composition of the...
- 11M.2.hl.TZ1.9a.ii: Deduce the equations for the formation of the major product at the positive electrode (anode)...
- 11M.2.hl.TZ1.9b.i: Use Table 14 of the Data Booklet to deduce the equation for the spontaneous reaction...
- 11M.2.hl.TZ1.9b.ii: Calculate the standard potential for this cell.
- 11M.2.hl.TZ1.9b.iii: State the conditions necessary for the potential of the cell to equal that calculated in part...
- 11M.2.hl.TZ1.9c: Using the data below and data from Table 14 of the Data Booklet, predict and explain which...
- 11M.2.hl.TZ1.9d.i: Electrolysis is used in the electroplating of metals. The same amount of current is passed...
- 11M.2.hl.TZ1.9d.ii: For the \({\text{Sn(S}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{2}}}\) cell, suggest two...
- 11M.1.hl.TZ2.32: The standard electrode potentials for two metals are given...
- 11M.1.hl.TZ2.33: The same quantity of electricity was passed through separate molten samples of sodium...
- 11M.2.hl.TZ2.7a.i: Define standard electrode potential.
- 11M.2.hl.TZ2.7b.ii: Deduce which species can reduce \({\text{S}}{{\text{n}}^{4 + }}{\text{(aq)}}\) to...
- 11M.2.hl.TZ2.7b.iii: Deduce which species can reduce \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) to Sn(s) under...
- 11M.2.hl.TZ2.7c.i: Draw a labelled diagram of a voltaic cell made from an Fe (s) /...
- 11M.2.hl.TZ2.7c.ii: Deduce the equation for the chemical reaction occurring when the cell in part (c) (i) is...
- 11M.2.hl.TZ2.7a.ii: Explain the significance of the minus sign in \( - {\text{0.45 V}}\).
- 11M.2.hl.TZ2.7b.i: State the species which is the strongest oxidizing agent.
- 11M.2.hl.TZ2.7e.ii: Explain why an aqueous solution of sodium chloride cannot be used to obtain sodium metal by...
- 12M.1.hl.TZ2.31: Two electrolytic cells are connected in series and the same current passes through each cell....
- 12M.1.hl.TZ2.30: Consider the following standard electrode...
- 11N.1.hl.TZ0.31: Four electrolytic cells are constructed. Which cell would produce the greatest mass of metal...
- 11N.2.hl.TZ0.7d.i: Draw a diagram of the voltaic cell, labelling the positive and negative electrodes (cathode...
- 11N.2.hl.TZ0.7d.ii: Define the term standard electrode potential.
- 11N.2.hl.TZ0.7d.iii: Calculate the cell potential, in V, under standard conditions, using information from Table...
- 11N.2.hl.TZ0.7f: Chromium is often used in electroplating. State what is used as the positive electrode...
- 18M.1.hl.TZ1.29: What are the products of electrolysis when concentrated calcium bromide solution is...

