| Date | May 2014 | Marks available | 4 | Reference code | 14M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | State and Suggest | Question number | 6 | Adapted from | N/A |
Question
Oxidation and reduction can be defined in terms of electron transfer or oxidation numbers.
A reactivity series can be experimentally determined by adding the metals W, X, Y and Z to solutions of these metal ions. The following reactions were observed:
\({{\text{W}}^{2 + }}{\text{(aq)}} + {\text{X(s)}} \to {\text{W(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{W(s)}}\)
\({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{W(s)}} \to {\text{Z(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{X(s)}}\)
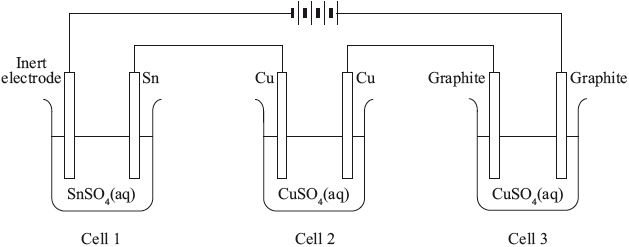
A student carries out the electrolysis of aqueous potassium iodide, KI, using inert electrodes.
Three electrolytic cells were set up in series (one cell after the other), as shown below.
All of the solutions had a concentration of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Alcohols with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) occur as four structural isomers. Three of the isomers can be oxidized with acidified potassium dichromate solution to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\).
(i) Deduce the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(ii) Deduce the overall equation for the redox reaction.
(iii) Two of the isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) can be oxidized further to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). Deduce the structural formulas of these two isomers.
(iv) One isomer cannot be oxidized by acidified potassium dichromate solution.
Deduce its structural formula, state its name and identify it as a primary, secondary or tertiary alcohol.
Name:
Alcohol:
(v) All isomers of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) undergo complete combustion. State an equation for the complete combustion of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(i) Deduce the order of reactivity of these four metals, from the least to the most reactive.
(ii) A voltaic cell is made by connecting a half-cell of X in \({\text{XC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\) to a half-cell of Z in \({\text{ZC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\). Deduce the overall equation for the reaction taking place when the cell is operating.
(iii) The standard electrode potential for \({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Z(s)}}\) is \( + {\text{0.20 V}}\). State which species is oxidized when this half-cell is connected to a standard hydrogen electrode.
(iv) Describe the standard hydrogen electrode including a fully labelled diagram.
(i) State the half-equation for the reaction that occurs at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(ii) Suggest, giving a reason, what would happen if the electrodes were changed to aluminium.
(i) Determine the mass of copper produced at one of the electrodes in cell 2 if the tin electrode in cell 1 decreased in mass by 0.034 g.
(ii) Compare the colour and the pH of the solutions in cells 2 and 3 after the current has been flowing for one hour.
(iii) Explain your answer given for part (d) (ii).
Colour:
pH:
Markscheme
(i) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} \to {{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Ignore state symbols.
(ii) \({\text{3}}{{\text{C}}_4}{{\text{H}}_9}{\text{OH(l)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{3}}{{\text{C}}_4}{{\text{H}}_8}{\text{O(l)}} + {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
(iii) \({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{OH}}\);
\({{\text{(C}}{{\text{H}}_3}{\text{)}}_2}{\text{CHC}}{{\text{H}}_2}{\text{OH}}\);
Accept full or condensed structural formulas.
(iv) \({{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}}\);
2-methylpropan-2-ol;
Allow 2-methyl-2-propanol, methylpropan-2-ol, methyl-2-propanol.
tertiary;
(v) \({{\text{C}}_4}{{\text{H}}_9}{\text{OH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}/{{\text{(C}}{{\text{H}}_3}{\text{)}}_3}{\text{COH}} + {\text{6}}{{\text{O}}_2} \to {\text{4C}}{{\text{O}}_2} + {\text{5}}{{\text{H}}_2}{\text{O}}\)
correct reactants and products;
correct balancing;
(i) \({\text{Z}} < {\text{W}} < {\text{X}} < {\text{Y}}\);
Accept Y > X > W > Z.
(ii) \({\text{X(s)}} + {{\text{Z}}^{2 + }}{\text{(aq)}} \to {{\text{X}}^{2 + }}{\text{(aq)}} + {\text{Z(s)}}\);
Ignore state symbols.
Accept X(s) + ZCl2(aq) \( \to \) XCl2(aq) + Z(s).
(iii) \({{\text{H}}_{\text{2}}}{\text{(g)}}\)/hydrogen;
(iv) diagram showing gas, solution and solid electrode;
For example,
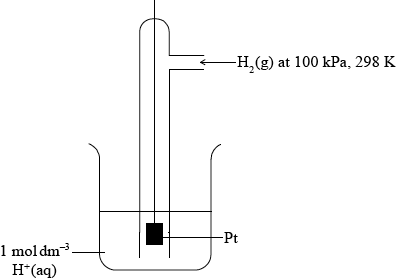
This diagram scores [3].
\({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ }}{{\text{H}}^ + }{\text{(aq)}}\) and Pt;
Allow 1 mol L–1 or 1 M.
Allow 1 mol dm–3 HCl (aq) or other source of 1mol dm–3 H+(aq) ions.
100 kPa/\({\text{1}}{{\text{0}}^{\text{5}}}{\text{ Pa}}\)/1 bar (\({{\text{H}}_2}{\text{(g)}}\) pressure) and 298K / 25 °C;
Ignore state symbols throughout.
Allow 1.01 \( \times \) 105 Pa/1 atm.
(i) Positive electrode (anode):
\({{\text{I}}^ - }{\text{(aq)}} \to \frac{1}{2}{{\text{I}}_2}{\text{(aq)}} + {{\text{e}}^ - }\);
Accept correct equation involving 2 mols of I–.
Negative electrode (cathode):
\({{\text{H}}_2}{\text{O(l)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}/{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}}/\)
\({{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {{\text{H}}_2}{\text{O(l)}} + \frac{1}{2}{{\text{H}}_2}{\text{(g)}}\);
Award [1 max] if correct equations are given at the wrong electrodes.
Ignore state symbols.
Allow e instead of e–.
Penalize equilibrium sign once only.
Accept correct equation involving 2 mols of H+.
(ii) aluminium will be oxidized (instead of \({{\text{I}}^ - }\)) at positive electrode (anode);
aluminium is a reactive metal / oxidation of aluminium has a positive \({E^\Theta }\) /
aluminium is higher on the reactivity series than \({{\text{I}}^ - }\) / OWTTE;
(i) \({{\text{n}}_{{\text{Sn}}}} = {{\text{n}}_{{\text{Cu}}}} = 2.86 \times {10^{ - 4}}/0.000286{\text{ (mol)}}\);
\({\text{m(Cu)}} = 2.86 \times {10^{ - 4}} \times 63.55 = 0.0182{\text{ (g)}}\);
(ii) blue colour persists in second cell and fades in third cell;
pH does not change in second cell and decreases in third cell;
Award [1 max] if both colour and pH are correctly stated for one only of either second or third cell.
(iii) Colour:
positive Cu electrode (anode) is oxidized to maintain colour in second cell / \({\text{Cu(s)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
pH:
in third cell, \({{\text{H}}^ + }\) ions are produced as water is oxidized at positive electrode (anode) / \({{\text{H}}_2}{\text{O(l)}} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\) / solution becomes acidic as hydroxide ions are oxidized at positive electrode (anode) / \({\text{2O}}{{\text{H}}^ - }{\text{(aq)}} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - }\);
Ignore state symbols.
Examiners report
Most candidates attempted writing half reactions, but few got them correct in a(i), a(ii), b(ii), c(i) and d(iii). The structure and conditions for the standard hydrogen electrode produced a range of marks, and there was a significant minority who drew two half cells. The importance of the nature of the electrodes was hardly ever explained, if comments about the inert nature of aluminium due to its oxide coating were mentioned they would have been given credit. Once again the inability to describe and then explain the processes happening during the electrolysis of copper sulphate was apparent.
Most candidates attempted writing half reactions, but few got them correct in a(i), a(ii), b(ii), c(i) and d(iii). The structure and conditions for the standard hydrogen electrode produced a range of marks, and there was a significant minority who drew two half cells. The importance of the nature of the electrodes was hardly ever explained, if comments about the inert nature of aluminium due to its oxide coating were mentioned they would have been given credit. Once again the inability to describe and then explain the processes happening during the electrolysis of copper sulphate was apparent.
Most candidates attempted writing half reactions, but few got them correct in a(i), a(ii), b(ii), c(i) and d(iii). The structure and conditions for the standard hydrogen electrode produced a range of marks, and there was a significant minority who drew two half cells. The importance of the nature of the electrodes was hardly ever explained, if comments about the inert nature of aluminium due to its oxide coating were mentioned they would have been given credit. Once again the inability to describe and then explain the processes happening during the electrolysis of copper sulphate was apparent.
Most candidates attempted writing half reactions, but few got them correct in a(i), a(ii), b(ii), c(i) and d(iii). The structure and conditions for the standard hydrogen electrode produced a range of marks, and there was a significant minority who drew two half cells. The importance of the nature of the electrodes was hardly ever explained, if comments about the inert nature of aluminium due to its oxide coating were mentioned they would have been given credit. Once again the inability to describe and then explain the processes happening during the electrolysis of copper sulphate was apparent.

