| Date | May 2011 | Marks available | 3 | Reference code | 11M.2.hl.TZ1.9 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Identify and State and explain | Question number | 9 | Adapted from | N/A |
Question
The conditions used in an electrolytic cell can determine the products formed.
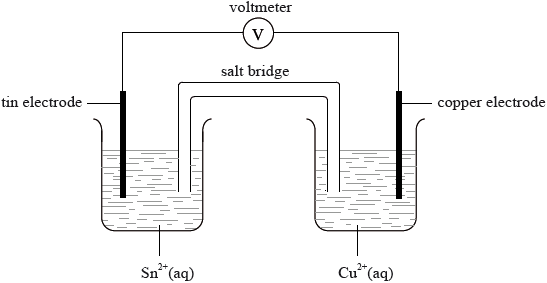
A voltaic cell is constructed from two half-cells as illustrated below.
Nitrogen monoxide may be removed from industrial emissions via a reaction with ammonia as shown by the equation below.
\[{\text{4N}}{{\text{H}}_3}{\text{(g)}} + {\text{6NO(g)}} \to {\text{5}}{{\text{N}}_2}{\text{(g)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Draw an electrolytic cell illustrating the electrolysis of molten nickel(II) bromide, \({\text{NiB}}{{\text{r}}_{\text{2}}}\). Include in the diagram the direction of the electron flow, the polarity of electrodes and state the half-equations for the product formed at each electrode.
Deduce the equations for the formation of the major product at the positive electrode (anode) when the following aqueous solutions are electrolysed.
• dilute sodium chloride
• concentrated sodium chloride
Use Table 14 of the Data Booklet to deduce the equation for the spontaneous reaction occurring in this cell.
Calculate the standard potential for this cell.
State the conditions necessary for the potential of the cell to equal that calculated in part (b) (ii) using the data from Table 14.
Using the data below and data from Table 14 of the Data Booklet, predict and explain which metal, cadmium or chromium, may be obtained by electrolysis of separate aqueous solutions of \({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}}\) ions and \({\text{C}}{{\text{r}}^{2 + }}{\text{(aq)}}\) ions.
Electrolysis is used in the electroplating of metals. The same amount of current is passed through separate aqueous solutions of \({\text{NiS}}{{\text{O}}_{\text{4}}}\), \({\text{Sn(S}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{2}}}\) and \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\) in separate electrolytic cells for the same amount of time. State and explain which cell would deposit the greatest amount (in mol) of metal. Identify the electrode at which the metal is deposited.
For the \({\text{Sn(S}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{2}}}\) cell, suggest two factors, other than time and current, that would affect the amount of metal deposited during electroplating.
Deduce the oxidation number of the nitrogen in the reactants and product.
Deduce the oxidation and reduction half-equations and identify the oxidizing agent for the reaction.
\({\text{30.0 d}}{{\text{m}}^{\text{3}}}\) of ammonia reacts with \({\text{30.0 d}}{{\text{m}}^{\text{3}}}\) of nitrogen monoxide at 100 °C. Identify which gas is in excess and by how much and calculate the volume of nitrogen produced.
Markscheme
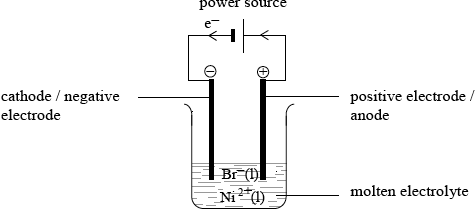
labelled polarities of positive and negative electrodes;
Electrodes can be labelled positive or negative or + and – signs can be used.
direction of electron flow;
\({e^ - }\) does not have to be labelled but arrow essential.
power source and molten electrolyte/\({\text{N}}{{\text{i}}^{2 + }}{\text{(l)}}\) and \({\text{B}}{{\text{r}}^ - }{\text{(l)/NiB}}{{\text{r}}_{\text{2}}}{\text{(l)}}\);
State symbol necessary for M3 unless molten electrolyte stated.
Power source does not need to be labelled if correct symbol used (i.e. short line and long line).
Cathode/negative electrode equation:
\({\text{N}}{{\text{i}}^{2 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{Ni}}\);
Anode/positive electrode equation:
\({\text{2B}}{{\text{r}}^ - } \to {\text{B}}{{\text{r}}_2} + {\text{2}}{{\text{e}}^ - }\);
Accept balanced half-equation with one \({e^ - }\).
Award [1 max] for M4 and M5 if electrodes are not identified or if equations are given wrong way round or incorrectly labelled.
Penalize \( \rightleftharpoons \) once only in Q.9.
Allow e instead of \({e^ - }\).
Ignore state symbols for M4 and M5.
Dilute sodium chloride:
\({\text{2}}{{\text{H}}_2}{\text{O}} \to {{\text{O}}_2} + {\text{4}}{{\text{H}}^ + } + {\text{4}}{{\text{e}}^ - }/{\text{4O}}{{\text{H}}^ - } \to {{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} + {\text{4}}{{\text{e}}^ - }\);
Concentrated sodium chloride:
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }\);
Accept alternative balanced half-equations with correct number of electrons.
Award [1 max] if equations are given the wrong way round.
Award [2] if correct equations are written in order with dilute sodium chloride first and concentrated sodium chloride second but processes not stated explicitly.
Penalize \( \rightleftharpoons \) once only in Q.9.
Allow e instead of \({e^ - }\).
Ignore state symbols.
\({\text{Sn}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{S}}{{\text{n}}^{2 + }} + {\text{Cu}}\);
Ignore state symbols.
Penalize \( \rightleftharpoons \) once only in Q.9.
\((0.34 - - 0.14) = ( + )0.48{\text{ V}}\);
\({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solutions and 25 °C/298 K;
\({\text{C}}{{\text{d}}^{2 + }}\) is a stronger oxidizing agent than \({{\text{H}}_{\text{2}}}{\text{O}}\) and will be displaced to produce Cd / OWTTE;
\({\text{C}}{{\text{r}}^{2 + }}\) is a weaker oxidizing agent than \({{\text{H}}_{\text{2}}}{\text{O}}\) and H2 will displace in preference to Cr / OWTTE;
Award [1 max] for stating Cd2+ stronger oxidizing agent than H2O and Cr2+ weaker oxidizing agent than H2O / OWTTE.
Ni;
only requires 2 mol of \({{\text{e}}^ - }\) for each mol of Ni / Sn requires 4 mol of \({{\text{e}}^ - }\) / Cr requires 3 mol of \({{\text{e}}^ - }\) / \({\text{N}}{{\text{i}}^{2 + }}\) needs least number of e– to produce 1 mol of Ni metal;
Allow e instead of \({e^ - }\).
cathode / negative electrode;
Do not award M3 for “metal deposited at cathode where oxidation occurs”.
temperature of solution;
\({\text{[S}}{{\text{n}}^{4 + }}{\text{]}}\);
surface area/size of electrode;
material of electrodes;
Do not allow nature of electrodes.
\({\text{N}}{{\text{H}}_{\text{3}}}\): \( - 3\);
NO: \( + 2\);
\({{\text{N}}_{\text{2}}}\): 0;
Penalize incorrect notation such as 3–, III, 2+, 2, II once only.
Oxidation:
\({\text{2N}}{{\text{H}}_{\text{3}}} \to {{\text{N}}_{\text{2}}} + {\text{6}}{{\text{H}}^ + } + {\text{6}}{{\text{e}}^ - }\);
Reduction:
\({\text{2NO}} + {\text{4}}{{\text{H}}^ + } + {\text{4}}{{\text{e}}^ - } \to {{\text{N}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}}\);
Award [1 max] for M1 and M2 if redox processes are not identified or if equations are given wrong way round.
Penalize \( \rightleftharpoons \) once only in Q.9.
Allow e instead of \({e^ - }\).
Ignore state symbols.
Oxidizing agent: NO;
Allow either formula or name.
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia (in excess by) and 10 (\({\text{d}}{{\text{m}}^{\text{3}}}\));
25.0 (\({\text{d}}{{\text{m}}^{\text{3}}}\));
Examiners report
This was also a popular question but candidates often struggled to do well. In (a) (i), a number of candidates confused this question with one on voltaic cells and drew two half-cells connected rather than that of an electrolytic cell for the electrolysis of nickel bromide. The half-equations on the whole were poor and most were unlabelled. Use of equilibrium signs was widespread and many candidates did not realise that reduction takes place at the cathode leading to the formation of Ni etc.
Few correctly answered correctly the equation for dilute solutions in (ii).
In (b) (i) most candidates got the correct equation though \({\text{C}}{{\text{u}}^ + }\) was often given.
In (ii) some candidates forgot to include V as the unit.
Few scored one mark in (iii).
Most candidates did not have any clue about part (c). Few spotted that they needed to compare the oxidizing/reducing power to that of water. Most simply made a comparison between the two electrode potentials given. Most candidates scored zero on this question.
(d) (i) was often well answered though many did not state that 2 mol of electrons are required for each mol of Ni.
(ii) proved difficult and there were a number of G2 comments stating that this went somewhat beyond the syllabus. These points were valid and this was taken into account during Grade Award.
In (e), although most candidates scored full marks, incorrect notations such as 3-, III were sometimes seen.
In contrast to (e) (i) both (ii) and (iii) were very well answered.
In contrast to (e) (i) both (ii) and (iii) were very well answered.

