| Date | May 2010 | Marks available | 4 | Reference code | 10M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Describe | Question number | 6 | Adapted from | N/A |
Question
The standard electrode potentials for three other electrode systems are given below.

Using Table 14 of the Data Booklet, calculate the cell potential for this cell.
The standard electrode potentials for three other electrode systems are given below.

(i) Identify which species in the table above is the best reducing agent.
(ii) Deduce the equation for the overall reaction with the greatest cell potential.
These values were obtained using a standard hydrogen electrode. Describe the materials and conditions used in the standard hydrogen electrode. (A suitably labelled diagram is acceptable).
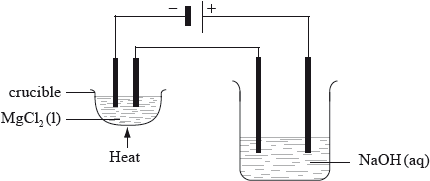
Two electrolytic cells are connected in series as shown in the diagram below. In one there is molten magnesium chloride and in the other, dilute sodium hydroxide solution. Both cells have inert electrodes. If 12.16 g of magnesium is produced in the first cell, deduce the identity and mass of products produced at the positive and negative electrodes in the second cell.
Markscheme
\( + 0.80 - ( - 2.37) = 3.17{\text{ V}}\)
correct data;
answer with unit;
Award [1] for –3.17 V or correct working of wrong values.
(i) Cd/Cd(s);
Do not allow Cd2+.
(ii) \({\text{5Cd(s)}} + {\text{2MnO}}_4^ - {\text{(aq)}} + {\text{16}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
balancing;
If wrong equation is given, award [1] for correct balancing.
Ignore state symbols.
Accept suitable diagram with the following indicated:
Pt electrode;
\({\text{[}}{{\text{H}}^ + }{\text{(aq)]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{/0.5 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\);
Accept Molar, M or mol L–1 instead of mol\(\,\)dm–3.
\({{\text{H}}_{\text{2}}}\) gas;
at 1 atm / \(1.01 \times {10^5}{\text{ Pa}}\);
Do not award mark for pressure if no hydrogen gas given.
Products:
oxygen at positive electrode and hydrogen at negative electrode;
Moles of \({\text{Mg}} = 0.5\) / mole ratio of \({{\text{O}}_{\text{2}}}:{{\text{H}}_{\text{2}}}\) is \(1:2\);
Can be implied by calculation.
mass oxygen \( = \left( {\frac{1}{2} \times \frac{{12.16}}{{24.31}} \times 32.00 = } \right){\text{ }}8.00{\text{ g}}\);
mass hydrogen \( = \left( {\frac{{12.16}}{{24.31}} \times 2.02 = } \right){\text{ }}1.01{\text{ g}}\);
Do not apply SD rule here.
Examiners report
Attempts at the equations were disappointing, with many written the wrong way round, or not identified as oxidation or reduction and some candidates gave equilibrium signs.
In (b)(i) the most common mistake was stating \({\text{C}}{{\text{d}}^{2 + }}\) instead of Cd. In (b) (ii) many candidates were tempted to write the more familiar equation involving \({\text{F}}{{\text{e}}^{2 + }}\) and \({\text{MnO}}_4^ - \) ions even if they identified Cd.
The hydrogen electrode did not seem to be well known, with few diagrams resembling the real thing, rarely were all 4 marks scored – often candidates mentioned 1 atm but not \({{\text{H}}_{\text{2}}}{\text{(g)}}\), or \({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) but not \({{\text{H}}^ + }\).
The calculation was poorly done - in most cases it involved the wrong products, which usually included sodium, very few candidates realised that the products would be hydrogen and oxygen.

