| Date | May 2017 | Marks available | 3 | Reference code | 17M.2.hl.TZ1.3 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Comment | Question number | 3 | Adapted from | N/A |
Question
Vanadium has a number of different oxidation states.
Electrode potentials for the reactions of vanadium and other species are shown below.
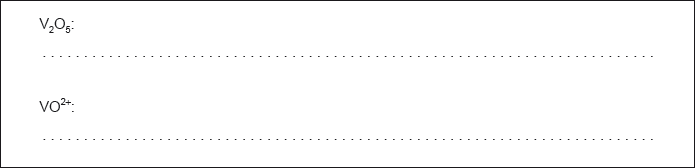
Determine the oxidation state of vanadium in each of the following species.
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
Identify, from the table, a non-vanadium species that could convert \({\text{VO}}_2^ + {\text{(aq)}}\) to V2+(aq).
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Comment on the spontaneity of this reaction by calculating a value for \(\Delta {G^\theta }\) using the data given in (b) and in section 1 of the data booklet.
Markscheme
\({V_2}{O_5}:{\text{ }} + 5\)
\(V{O^{2 + }}:{\text{ }} + 4\)
Do not penalize incorrect notation twice.
[2 marks]
H2SO3(aq)
OR
Pb(s)
[1 mark]
Zn(s)
[1 mark]
\({\text{V}}{{\text{O}}^{2 + }}({\text{aq)}} + {{\text{V}}^{2 + }}({\text{aq)}} + {\text{2}}{{\text{H}}^ + }({\text{aq)}} \to {\text{2}}{{\text{V}}^{3 + }}({\text{aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Accept equilibrium sign.
[1 mark]
\({E^\theta }\ll = + 0.34{\text{ V}} - ( - 0.26{\text{ V}})\gg = + 0.60{\text{ }}\ll {\text{V}}\gg \)
\(\Delta {G^\theta } = \ll - nF{E^\theta } = - 9.65 \times {10^4}{\text{ C}}\,{\text{mo}}{{\text{l}}^{ - 1}} \times 0.60{\text{ J}}\,{{\text{C}}^{ - 1}} = \gg - 57\,900{\text{ }}\ll {\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}\gg / - 57.9{\text{ }}\ll {\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\gg \)
spontaneous as \(\Delta {G^\theta }\) is negative
Do not award M3 as a stand-alone answer.
Accept “spontaneous” for M3 if answer given for M2 is negative.
Accept “spontaneous as \({E^\theta }\) is positive” for M3.
[3 marks]

