| Date | November 2012 | Marks available | 16 | Reference code | 12N.2.hl.TZ0.4 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Describe, Draw, and Identify | Question number | 4 | Adapted from | N/A |
Question
Arsenic and nitrogen play a significant role in environmental chemistry. Arsenous acid, H3AsO3, can be found in oxygen-poor (anaerobic) water, and nitrogen-containing fertilizers can contaminate water.
The electrolysis of aqueous copper(II) sulfate is an example of an electrolysis process where the nature of the electrodes can determine which products form. Platinum electrodes were used in process 1 and copper electrodes in process 2.
(i) Draw an annotated diagram of the electrolytic cell in process 1 and identify the direction of electron flow.
(ii) For process 1 (platinum electrodes), state the half-equations occurring at the positive electrode (anode) and negative electrode (cathode). Include state symbols for all species. Describe what is observed at each electrode and comment on any change in the colour and the acidity of the solution.
Half-equation at positive electrode (anode):
Half-equation at negative electrode (cathode):
Observation at positive electrode (anode):
Obsevation at negative electrode (cathode):
Change in colour (if any) of the solution:
Change in acidity (if any) of the solution:
(iii) For process 2 (copper electrodes), state the half-equations occurring at the positive electrode (anode) and negative electrode (cathode). Include state symbols for all species. Describe what is observed at each electrode and comment on any change in the colour and the acidity of the solution.
Half-equation at positive electrode (anode):
Half-equation at negative electrode (cathode):
Observation at positive electrode (anode):
Observation at negative electrode (cathode):
Change in colour (if any) of the solution:
Change in acidity (if any) of the solution:
Markscheme
(i) Diagram to show:
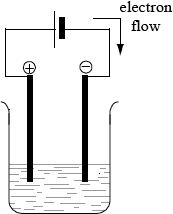
Labels are not required.
one container, two electrodes, battery (and electrolyte);
Allow + and – for representation of battery (could be long and short lines for example) but M1 is not scored if a voltmeter/V if shown or labelled.
Ignore designation of electrodes (e.g. do not penalize Cu electrodes).
correct direction of electron flow (from negative pole to positive pole);
Allow arrow without stating e– explicitly.
To score M2, the polarity of the battery or the cathode and anode must be shown.
If a voltmeter/V is shown, M1 is not awarded but M2 may be scored if the cathode and anode are identified with the correct direction of electron flow.
(ii) Half-equation at positive electrode (anode):
\({\text{4O}}{{\text{H}}^ - }{\text{(aq)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{e}}^ - }/{\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - }{\text{/}}\)
\({\text{2O}}{{\text{H}}^ - }{\text{(aq)}} \to \frac{1}{2}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - }\);
Allow e instead of e–.
Half-equation at negative electrode (cathode):
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Cu(s)}}\);
Allow e instead of e–.
Award [1 max] for M1 and M2 if correct equations are given but at wrong electrodes.
Penalize \( \rightleftharpoons \) once only in (b)(ii) and (b)(iii).
correct state symbols in all equations;
M3 can only be scored if the correct species are given in M1 and M2 (i.e. do not award ECF from M1 and M2 for incorrect species).
Observation at positive electrode (anode):
bubbles / gas;
Award mark for observation even if type of gas is incorrect (e.g. hydrogen).
Observation at negative electrode (cathode):
red/brown/copper/metal (deposit);
Allow mass increases / gets thicker/larger / OWTTE.
Change in colour (in any) of the solution:
solution loses blue colour/becomes paler;
Change in acidity (if any) of the solution:
becomes (more) acidic / pH decreases;
(iii) Half-equation at positive electrode (anode):
\({\text{Cu(s)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Allow e instead of e–.
Half-equation at negative electrode (cathode):
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Cu(s)}}\);
Allow e instead of e–.
Award [1 max] for M1 and M2 if correct equations are given but at wrong electrodes.
Penalize \( \rightleftharpoons \) once only in (b)(ii) and (b)(iii).
correct state symbols in all equations;
M3 can only be scored if the correct species are given in M1 and M2 (i.e. do not award ECF from M1 and M2 for incorrect species).
Observation at positive electrode (anode):
(slowly) dissolves / OWTTE;
Allow mass decreases / gets smaller/thinner / OWTTE;
Accept impurities deposited under positive electrode/anode.
Observation at negative electrode (cathode):
red/brown/copper/metal deposit;
Allow mass increases / gets thicker/larger / OWTTE.
Change in colour (if any) of the solution:
blue colour remains;
Allow no change or solution remains same colour.
Change in acidity (if any) of the solution:
solution does not become (more) acidic / no change / OWTTE;
Examiners report
In part (b), the most common mistake involved the incorrect direction of electron flow. Some candidates also failed to include a battery for the electrolytic cell. The weaker students drew voltaic cells with salt bridges. In part (ii) and part (iii) the very best students often scored full marks. However, a significant majority of candidates only gained partial marks on these two parts and the correct half-equation at the anode in part (ii) was particularly troublesome. Other mistakes included writing equilibrium signs and half-equations involving platinum which showed weak overall understanding of the two processes.

