| Date | November 2009 | Marks available | 2 | Reference code | 09N.2.hl.TZ0.4 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce | Question number | 4 | Adapted from | N/A |
Question
Consider the following half-cell reactions and their standard electrode potentials.
\[\begin{array}{*{20}{l}} {{\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Ni(s)}}}&{{E^\Theta } = - {\text{0.26 V}}} \\ {{\text{A}}{{\text{l}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Al(s)}}}&{{E^\Theta } = - {\text{1.66 V}}} \end{array}\]
Outline two differences between an electrolytic cell and a voltaic cell.
Deduce a balanced equation for the overall reaction which will occur spontaneously when these two half-cells are connected.
Determine the cell potential when the two half-cells are connected.
On the cell diagram below, label the negative electrode (anode), the positive electrode (cathode) and the directions of the movement of electrons and ion flow.
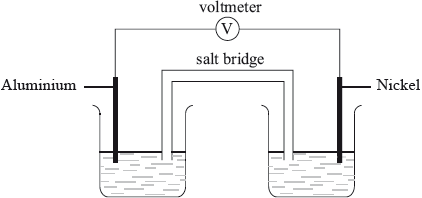
Markscheme
electrolytic cell converts electrical energy to chemical energy and voltaic cell converts chemical energy to electrical energy / electrolytic cell uses electricity to carry out a (redox) chemical reaction and voltaic cell uses a (redox) chemical reaction to produce electricity / electrolytic cell requires a power supply and voltaic cell does not;
electrolytic cell involves a non-spontaneous (redox) reaction and voltaic cell involves a spontaneous (redox) reaction;
in an electrolytic cell, cathode is negative and anode is positive and vice-versa for a voltaic cell / electrolytic cell, anode is positive and voltaic cell, anode is negative / electrolytic cell, cathode is negative and voltaic cell, cathode is positive;
voltaic cell has two separate solutions and electrolytic cell has one solution / voltaic cell has salt bridge and electrolytic cell has no salt bridge;
electrolytic cell, oxidation occurs at the positive electrode/anode and voltaic cell, oxidation occurs at the negative electrode/anode/vice-versa;
If descriptions are reversed for electrolytic and voltaic cell, penalize first marking point but award second marking point as ECF.
\({\text{2Al(s)}} + {\text{3N}}{{\text{i}}^{2 + }}{\text{(aq)}} \to {\text{2A}}{{\text{l}}^{3 + }}{\text{(aq)}} + {\text{3Ni (s)}}\);
Correct reactants and products, award [1]
Balancing award [1].
Ignore state symbols and equilibrium sign.
\(( + )1.40{\text{ (V)}}\);
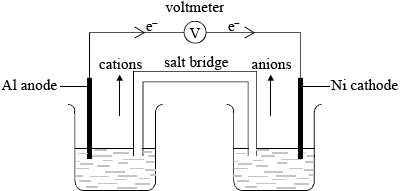
aluminium anode/negative electrode;
nickel cathode/positive electrode;
electron movement from Al to Ni;
correct movement of cations and anions through salt bridge;
If electron movement shown correctly but not labelled, award the mark
Examiners report
This question was generally well answered. The majority of candidates were able to state two differences between a voltaic and an electrolytic cell.
In part (b), a balanced equation and cell potential were correctly deduced by most candidates.
In part (b), a balanced equation and cell potential were correctly deduced by most candidates.
The ability to label the direction of the movement of cations and anions through the salt bridge proved to be the most difficult question on the paper.

