-1.png)
These slides summarise the essential understanding and skills in this topic.
They contain short explanations in text and images - good revision for all students.
Read the slides and look up any words or details you find difficult to understand.
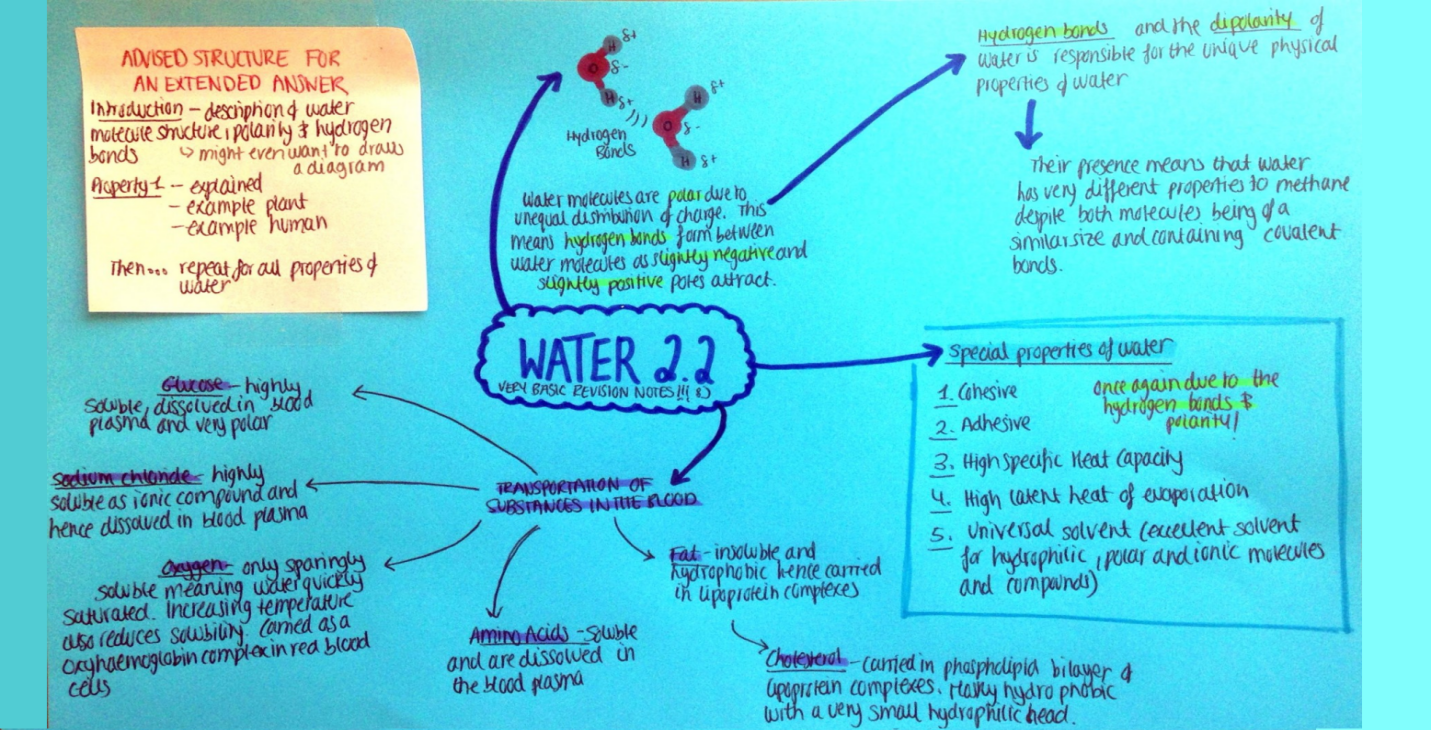
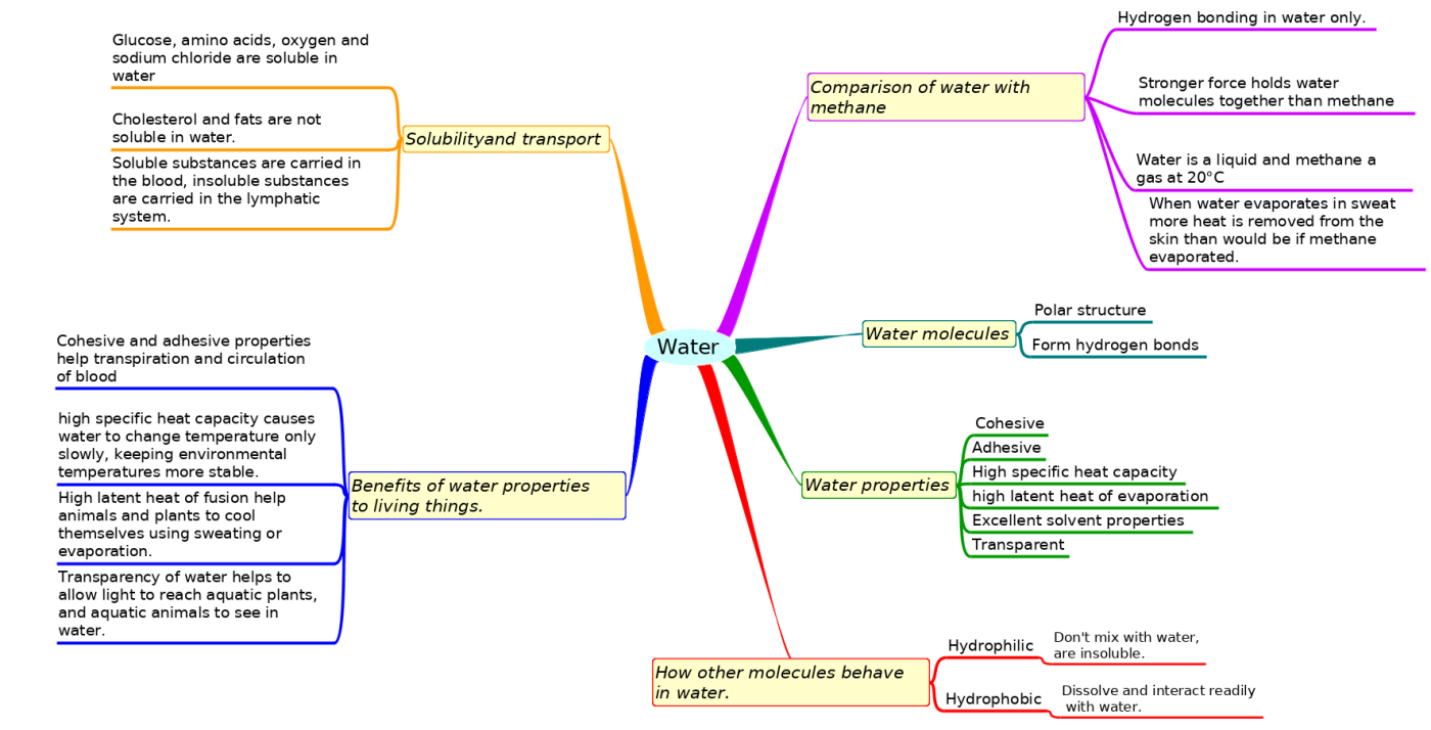
Summary list for 2.2 Water
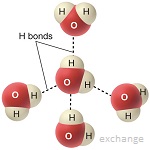
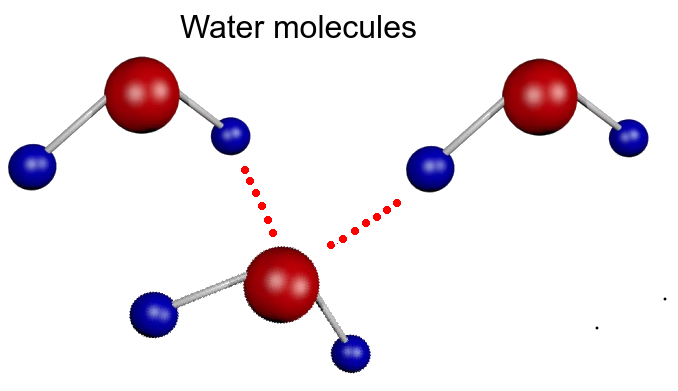
- Hydrogen bonds form between polar water molecules.
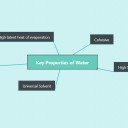
- This force give water special properties, e.g. cohesiveness, adhesiveness, thermal
and solvent properties. - Glucose, amino acids and salts are hydrophilic while cholesterol and fats are hydrophobic.
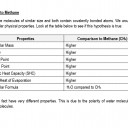
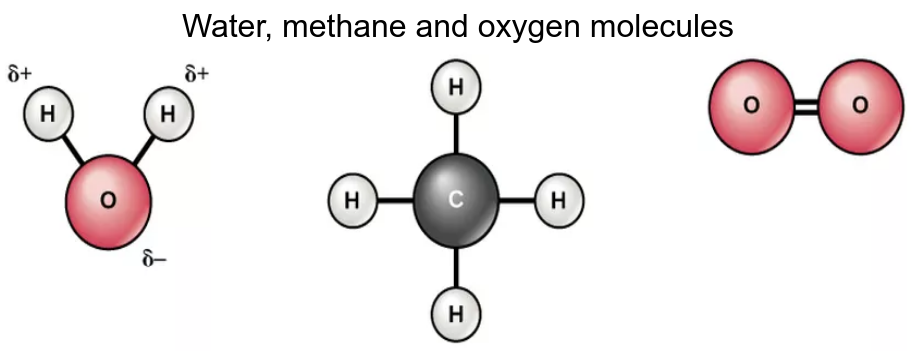
- Compare the thermal properties of water with those of methane and explain how this affects its use a as a coolant in sweat.
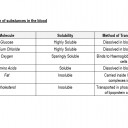
- Compare the solubility of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in water and link this to the way they are transported.
- Explain benefits of water properties to living organisms (Transparency and density not required).
- Evaluate how significant hydrogen bonding is in the properties of water
Mindmaps
These diagram summaries cover the main sections of topic 2.2 Water
Study them and draw your own list or concept map from memory.
Exam style question about hydrogen bonding
Understanding the way in which hydrogen bonding affects water molecules is an important skill from this topic.
Answer the question below on a piece of paper, then check your answer against the model answer below.
Outline the properties of water which result from hydrogen bonding between water molecules [4]
....................................................................................... ............................................................................
....................................................................................... ............................................................................
....................................................................................... ............................................................................
....................................................................................... ............................................................................
Click the + icon to see a model answer.
This is a self marking quiz containing questions covering the topic outlined above.
Try the questions to check your understanding.
START QUIZ!
Drag and drop activities
Test your ability to construct biological explanations using the drag and drop questions below.
Drag and drop learning engagements
Test your construction of biological knowledge using the drag and drop questions below.
Drag and drop the correct term into the gap to describe the importance of water properties to living systems.
raise carbohydrates Cohesion soil High sweat other heat capacity Solvent aquatic water stems hydrophilic
The polarity of the covalent bonds between the atoms in the water molecule causes hydrogen bonds between the molecules. These give the following properties which are vital to the functions of living organisms:
- , water molecules attract each other - allows an unbroken column to rise up the of tall trees.
- Adhesion, water molecules are attracted to charged molecules - retains water in .
- - dissolves molecules such as ions, proteins and in blood plasma.
- High , large quantity of heat needed to the temperature of water, stable environments.
- heat of vapourisation, large quantity of heat to evaporate - as a coolant.
Examiner hint - Use correct terminology. You need to know examples of how the properties of water are important to living systems.
energy solvent enzyme bilayers protective polar Amphoteric keratin Insoluble transported Lipids
The nature of the water molecule makes it a good for hydrophilic molecules. Water is the solvent in which most controlled reactions of the body occur and, in which, soluble substances are round the organism.
substances have important roles in storage and as structural components of cells and tissues. and starch are insoluble storage compounds, once formed inside a cell they cannot diffuse out of the cell. Insoluble proteins such as found in hair, horn and hooves have and locomotive functions.
phospholipids molecules assemble into forming the basis of the cell membrane.
Just for fun
Everyone needs a bit of fun while they revise. Try this Water wordshoot revision game. Can you reach the leader board?
How much of Water 2.2 have you understood?












 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn