Internal Energy
- When a substance gains or loses thermal energy, its internal energy increases or decreases
- The internal energy of a substance is defined as:
The sum of the total kinetic energy and the total intermolecular potential energy of the particles within the substance
- As thermal energy is transferred to a substance, two things can happen:
- An increase in the average kinetic energy of the molecules within the substance - i.e. the molecules vibrate and move at higher speeds
- An increase in the potential energy of the molecules within the substance - i.e. the particles get further away from each other or move closer to each other
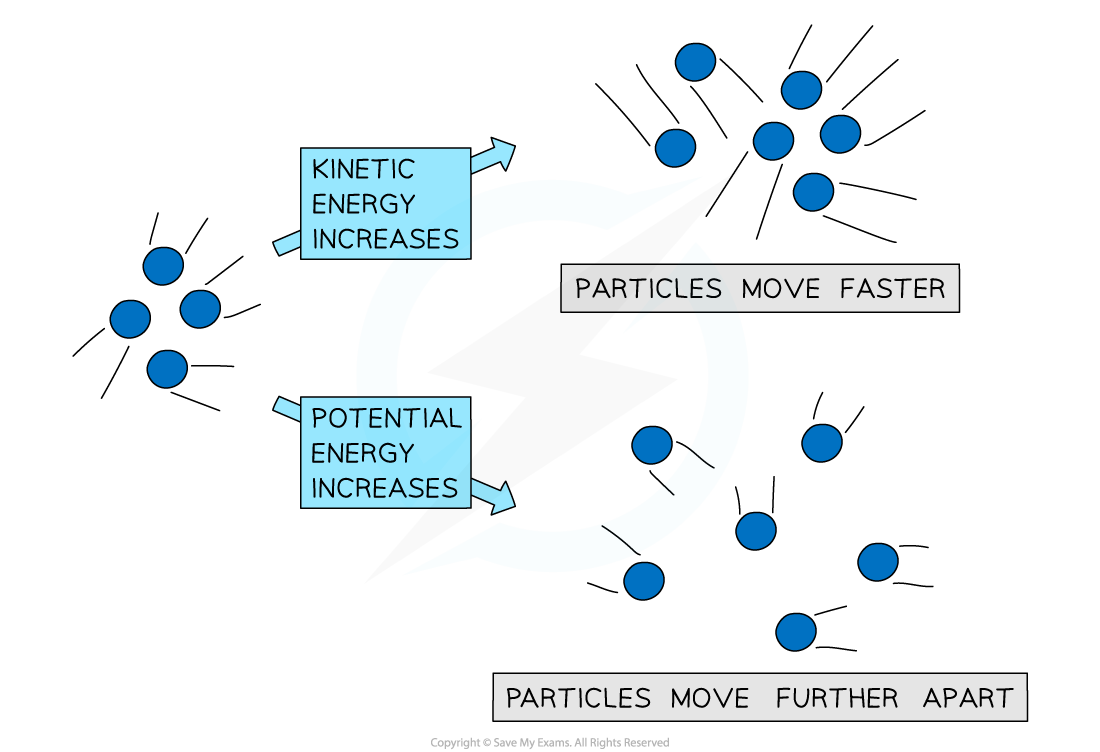
- Since temperature is a measure of the average kinetic energy of the molecules, only an increase in the average kinetic energy of the molecules will result in an increase in temperature of the substance
- Due to thermal expansion, when the temperature of a substance increases, the potential energy of the molecules also increases
- When only the potential energy of the molecules changes, the temperature of the substance does not change
- This is the case for all state changes (e.g. melting, boiling)
Exam Tip
Remember that a change in internal energy does not necessarily corresponds to a change in temperature.
- A change in the average kinetic energy of the molecules corresponds to a change in temperature
- A change in the average potential energy of the molecules does not affect temperature
