| Date | May 2021 | Marks available | 1 | Reference code | 21M.2.hl.TZ2.4 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify | Question number | 4 | Adapted from | N/A |
Question
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
State the hybridization of the carbon I and II atoms in but-2-ene.
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows.
Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
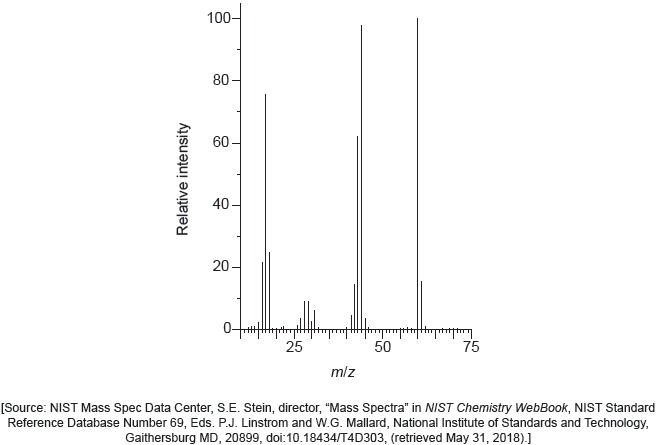
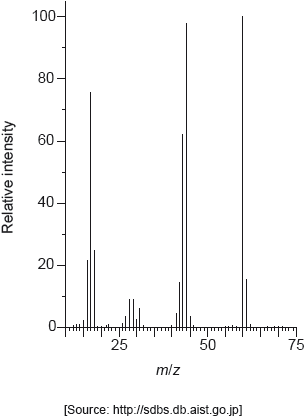
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
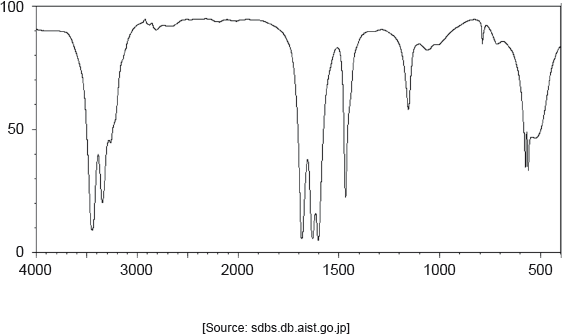
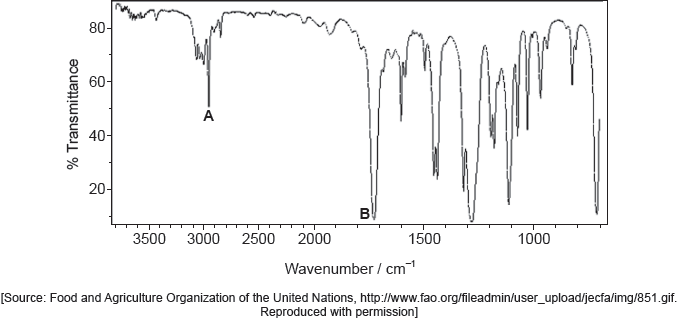
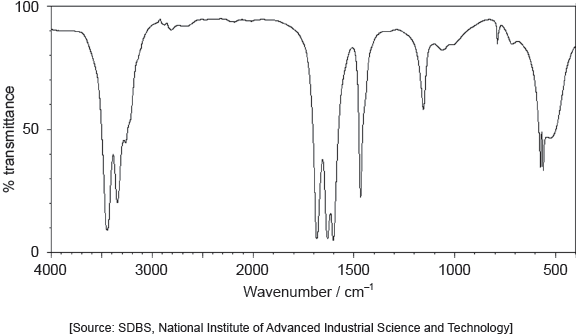
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
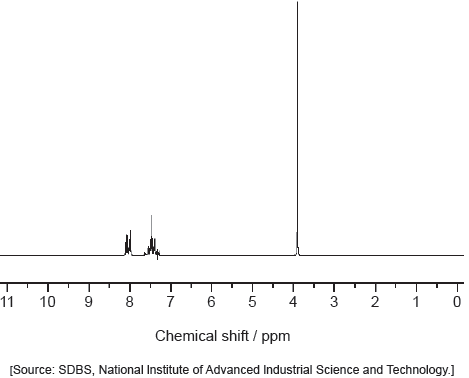
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
Outline how two enantiomers can be distinguished using a polarimeter.
Markscheme
Penalize missing hydrogens in displayed structural formulas once only.
Accept condensed structural formulas: CH3CH(OH)CH2CH3 / CH3CH2CH2CH3 or skeletal structures.
Bonds broken:
2(C–C) + 1(C=C) + 8(C–H) + 6O=O / 2(346) + 1(614) + 8(414) + 6(498) / 7606 «kJ» ✓
Bonds formed:
8(C=O) + 8(O–H) / 8(804) + 8(463) / 10 136 «kJ» ✓
Enthalpy change:
«Bonds broken – Bonds formed = 7606 kJ – 10 136 kJ =» –2530 «kJ» ✓
Award [3] for correct final answer.
Award [2 max] for «+» 2530 «kJ».
Sigma (σ):
Accept any diagram showing end to end/direct overlap of atomic/hybridized orbitals and electron density concentrated between nuclei.
Pi (π):
Accept any diagram showing sideways overlap of unhybridized p/atomic orbitals and electron density above and below plane of bond axis.
Alternative 1
Penalize incorrect bond e.g., -CH-H3C or –CH3C only once in the paper.
Alternative 2
curly arrow going from C=C to H of HBr AND curly arrow showing Br leaving ✓
representation of carbocation ✓
curly arrow going from lone pair/negative charge on Br− to C+ ✓
«2-bromo-2-methylbutane involves» formation of more stable «tertiary» carbocation/intermediate
OR
«2-bromo-3-methylbutane involves» formation of less stable «secondary» carbocation/intermediate ✓
«intermediate» more stable due to «increased positive» inductive/electron-releasing effect of extra –R/alkyl group/–CH3/methyl ✓
Do not award marks for quoting Markovnikov’s rule without any explanation.
m/z 58:
molar/«relative» molecular mass/weight/Mr «is 58 g mol−1/58» ✓
m/z 43:
«loses» methyl/CH3 «fragment»
OR
COCH3+ «fragment» ✓
Do not penalize missing charge on the fragments.
Accept molecular ion «peak»/ CH3COCH3+/C3H6O+.
Accept any C2H3O+ fragment/ CH3CH2CH2+/C3H7+.
C=O ✓
Accept carbonyl/C=C.
Information deduced from 1H NMR:
«one signal indicates» one hydrogen environment/symmetrical structure
OR
«chemical shift of 2.2 indicates» H on C next to carbonyl ✓
Compound:
propanone/CH3COCH3 ✓
Accept “one type of hydrogen”.
Accept .
enantiomers rotate «plane of» plane-polarized light ✓
equal degrees/angles/amounts AND opposite directions/rotation ✓
Accept “optical isomers” for “enantiomers”.