| Date | November 2020 | Marks available | 1 | Reference code | 20N.2.hl.TZ0.2 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Justify and Deduce | Question number | 2 | Adapted from | N/A |
Question
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR
State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
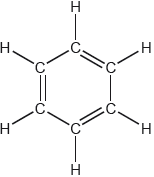
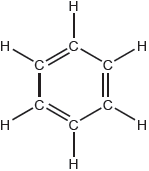
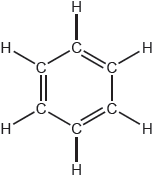
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
Markscheme
Electron domain geometry: tetrahedral ✔
Molecular geometry: bent/V-shaped ✔
✔
-bonds:
AND
-bonds: ✔
B AND absorption/
OR
B AND absence of ✔
Accept any value between .
Accept any two isomers except for propanone and propen-2-ol:
✔✔
Penalize missing hydrogens in displayed structural formulas once only.
AND is greater than /large ✔
✔
/water «and » ✔
/propan-2-ol ✔
/«potassium» dichromate(VI) AND
OR
/«acidified potassium» manganate(VII) ✔
Accept .
primary carbocation «intermediate forms»
OR
minor product «of the water addition would be» propan-1-ol
OR
anti-Markovnikov addition of water ✔
primary alcohol/propan-1-ol oxidizes to an aldehyde/propanal ✔
Examiners report
The majority of students got at least one of electron domain geometry or molecular geometry correct.
The vast majority of students could identify the hybridization around a central carbon atom.
The vast majority of students could identify BOTH sigma and pi bonds in a molecule.
Many candidates identified B having C = O and a peak at 1750.
A surprising number of candidates drew propanone here as an option, either failing to read the question or perhaps finding the structural formulae provided difficult to understand.
Most candidates identified B, the product, as being in greater concentration at equilibrium however some lost the mark because they did not include a reason.
Most candidates could apply the formula for Gibbs free energy change, ΔGΘ, correctly however some did not get the units correct.
The mean mark was ⅔ for the required synthetic route. Some candidates failed to identify water as a reagent in the hydration reaction, or note that dichromate ion oxidation requires acidic conditions. This was also the question with most No Response.
This question regarding the formation of a minor product was not well answered. Many candidates struggled to explain the formation of propan-1-ol and to then oxidize it to propanal.