| Date | May 2019 | Marks available | 2 | Reference code | 19M.2.sl.TZ2.1 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 1 | Adapted from | N/A |
Question
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
Product A contains a carbon–carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
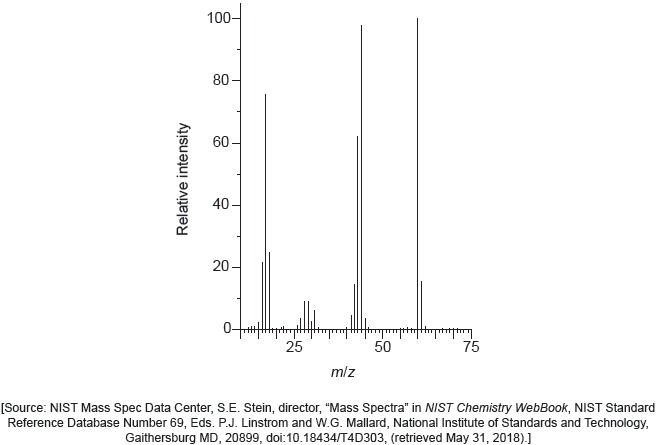
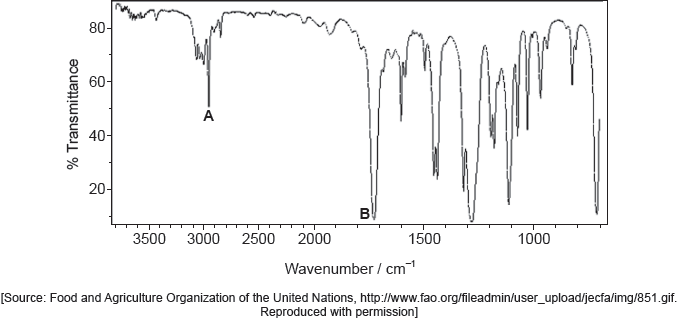
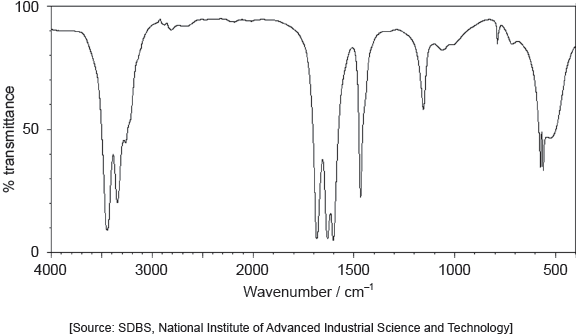
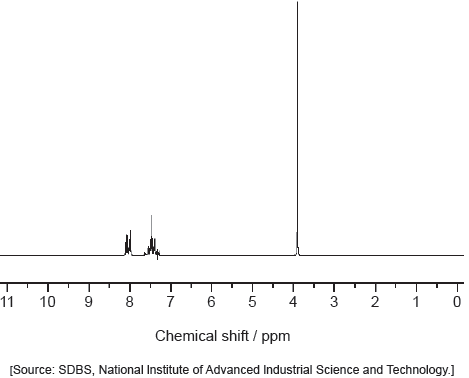
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Markscheme
C2H2 (g) + 2.5O2 (g) → 2CO2 (g) + H2O (l)
OR
2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O (l) [✔]
[✔]
Note: Accept any valid combination of lines, dots and crosses.
«ethyne» shorter AND a greater number of shared/bonding electrons
OR
«ethyne» shorter AND stronger bond [✔]
London/dispersion/instantaneous dipole-induced dipole forces [✔]
Note: Do not accept just “intermolecular forces” or “van der Waals’ forces”.
«electrophilic» addition/A«E» [✔]
Note: Accept “polymerization”.
ethanal [✔]
«sum of bond enthalpies of reactants =» 2(C–H) + C≡C + 2(O–H)
OR
2 × 414 «kJ mol–1» + 839 «kJ mol–1» + 2 × 463 «kJ mol–1»
OR
2593 «kJ» [✔]
«sum of bond enthalpies of A =» 3(C–H) + C=C + C–O + O–H
OR
3 × 414 «kJ mol–1» + 614 «kJ mol–1» + 358 «kJ mol–1» + 463 «kJ mol–1»
OR
2677 «kJ» [✔]
«enthalpy of reaction = 2593 kJ – 2677 kJ» = –84 «kJ» [✔]
Note: Award [3] for correct final answer.
B AND it has a more negative/lower enthalpy/«potential» energy
OR
B AND more exothermic «enthalpy of reaction from same starting point» [✔]
Identity of product: «B»
IR spectrum:
1700–1750 «cm–1 band» AND carbonyl/CO group present
OR
no «band at» 1620–1680 «cm–1» AND absence of double bond/C=C
OR
no «broad band at» 3200–3600 «cm–1» AND absence of hydroxyl/OH group [✔]
Note: Accept a specific value or range of wavenumbers and chemical shifts.
1H NMR spectrum:
«only» two signals AND A would have three
OR
«signal at» 9.4–10.0 «ppm» AND «H atom/proton of» aldehyde/–CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of alkyl/CH next to» aldehyde/CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of» RCOCH2- present
OR
no «signal at» 4.5–6.0 «ppm» AND absence of «H-atom/proton next to» double bond/C=C [✔]
Note: Accept “two signals with areas 1:3”.
Reagents:
acidified/H+ AND «potassium» dichromate«(VI)»/K2Cr2O7/Cr2O72– [✔]
Conditions:
distil «the product before further oxidation» [✔]
Note: Accept “«acidified potassium» manganate(VII)/KMnO4/MnO4–/permanganate”.
Accept “H2SO4” or “H3PO4” for “H+”.
Accept “more dilute dichromate(VI)/manganate(VII)” or “excess ethanol”.
Award M1 if correct reagents given under “Conditions”.
–1 [✔]
Any three of:
has an oxygen/O atom with a lone pair [✔]
that can form hydrogen bonds/H-bonds «with water molecules» [✔]
hydrocarbon chain is short «so does not disrupt many H-bonds with water molecules» [✔]
«large permanent» dipole-dipole interactions with water [✔]
Examiners report
Almost all candidates recognized the products of the complete combustion of ethyne, and over two thirds managed to balance the equation. It was good to see candidates using integers for the balancing.
The majority of candidates drew the Lewis structure of ethyne. A few teachers commented that they did not cover alkynes assuming they are not included in the syllabus. Please check the current syllabus carefully when preparing students.
A very well answered question. The vast majority of candidates understood that triple bonds are stronger than single bonds and result in a shorter bond length. It was disappointing, however, to see a considerable number of candidates stating that ethane has a double bond.
Some candidates could not relate evaporation of a liquid to the breaking of its intermolecular forces and gave irrelevant answers such as “evaporation”. Other candidates gave general answers such as “the intermolecular forces” or used the term “van der Waals’ forces” which did not gain credit as too vague. The current guide is clear that “London/dispersion forces” is the appropriate term to use for instantaneous dipole-induced dipole forces. Less than 40 % of the candidates scored the mark. It was disappointing to see some candidates state “covalent bonding” as the type of interaction that must be overcome when liquid ethyne vaporizes. Some teachers thought the wording of the question may have been vague and candidates may have been confused about what was meant by the “type of interaction”.
About 60 % of the candidates stated “addition” as the type of reactions that compounds containing carbon-carbon double bonds underwent. It was disappointing to see a variety of answers including substitution, condensation and combustion showing a total lack of understanding. Some candidates gave specific types such as "bromination" or “hydration” which did not receive the mark.
60 % of the candidates were able to name compound B as ethanal. Some candidates did not recognize it as an aldehyde and gave names related to carboxylic acids or other homologous series. Other candidates called it methanal.
Candidates were confident in using average bond enthalpies for calculating the enthalpy change for the reaction. Mistakes included forgetting to include the breaking of the O-H bonds in water and reversing the signs.
Reasonably well answered. About half of the candidates showed understanding of the relation between stability and the enthalpy change from the same starting materials. ECF was applied in this question based on the answer in part (iii).
The majority of candidates handled this question competently and nearly half of the candidates obtained both marks. They obtained the value of the absorption from the spectra provided and compared it to the values in the data booklet to deduce the identity of the product. Common mistakes included not identifying the peaks and signals precisely (for example C=O instead of CHO for 1H NMR signal at 9.4-10.0 ppm). Some teachers commented that the TMS signal should not have been included as the SL do not know about it. Other teachers commented that using the 'actual' rather than an ‘idealized’ IR spectrum may have caused confusion due to the peak at around 3400 cm-1 which could be confused for O-H in alcohols. Thankfully both of these answers were hardly seen in the scripts. The peak at 3400 cm-1 was not at all broad and did not confuse the majority of students. Please note that real spectra are usually used in examination papers, and it is worth encouraging students to check more than one peak to confirm their deductions.
Surprisingly, this question was not answered well by the majority of the candidates. However, it did discriminate well between high-scoring and low-scoring candidates. Common mistakes included incorrect formulas (such as K2CrO7), missing the acidic conditions and stating “reflux” instead of “distillation”. Many candidates gave completely irrelevant reagents and conditions such as “oxygen, pressure and a nickel catalyst”. It is possible that some candidates did not think of “distillation” as a “condition”.
About 60 % of the candidates determined the average oxidation state of carbon in ethanal. A couple of teachers commented that asking SL students to determine an “average oxidation state” seems a little difficult. Please note that this term has been used in recent papers whenever there are two or more atoms of the element in different parts of the compound. There was no evidence of confusion on the part of the candidates and most answered the question well.
This was a challenging question with a demanding markscheme. Most students missed the fact that ethanal can form hydrogen bonds with water. And students who did state this often achieved only 1 out of the 3 marks because they did not offer a full explanation. Some candidates stating "hydrogen bonding" showed confusion by mentioning the hydrogen of the aldehyde group. Few identified the lone pairs on oxygen as the reason for the ability to hydrogen bond. Most candidates just stated that ethanal is polar and dissolves in polar water achieving no marks. However, one mark was awarded for “dipole-dipole interactions with water”.