| Date | November 2021 | Marks available | 1 | Reference code | 21N.2.hl.TZ0.1 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Predict | Question number | 1 | Adapted from | N/A |
Question
A 4.406 g sample of a compound containing only C, H and O was burnt in excess oxygen. 8.802 g of CO2 and 3.604 g of H2O were produced.
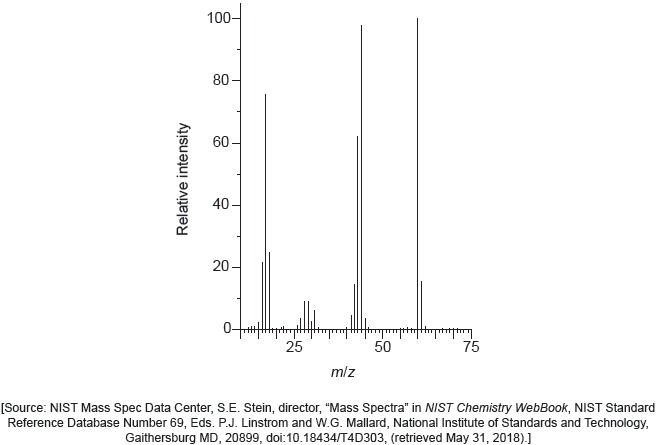
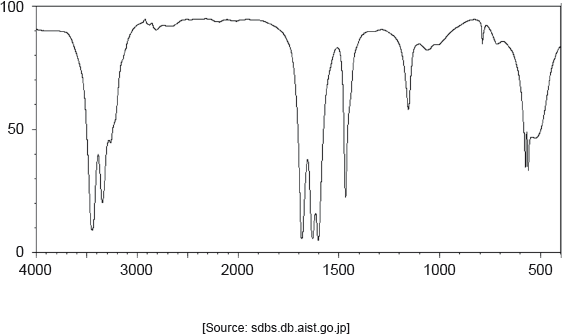
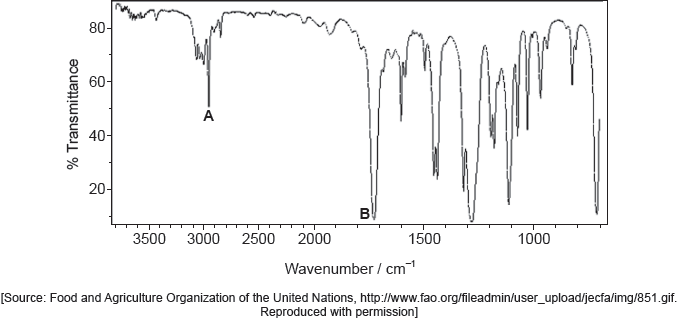
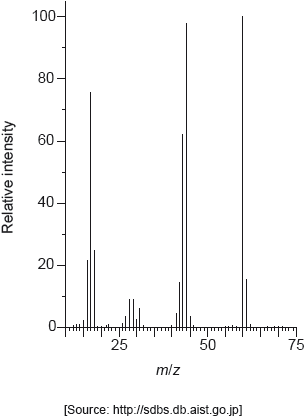
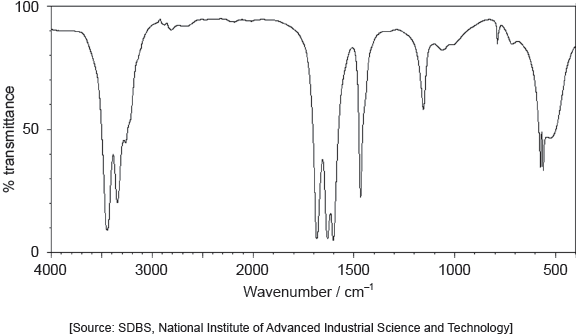
The following spectrums show the Infrared spectra of propan-1-ol, propanal and propanoic acid.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C71238&Units=SI&Type=IRSPEC&Index=3#IR-SPEC [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C79094&Units=SI&Mask=80#IR-Spec [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?Name=propanal&Units=SI&cIR=on&cTZ=on#IRSpec [Accessed 6 May 2020]. Source adapted.
Determine the empirical formula of the compound using section 6 of the data booklet.
Determine the molecular formula of this compound if its molar mass is 88.12 g mol−1. If you did not obtain an answer in (a) use CS, but this is not the correct answer.
Identify each compound from the spectra given, use absorptions from the range of 1700 cm−1 to 3500 cm−1. Explain the reason for your choice, referring to section 26 of the data booklet.
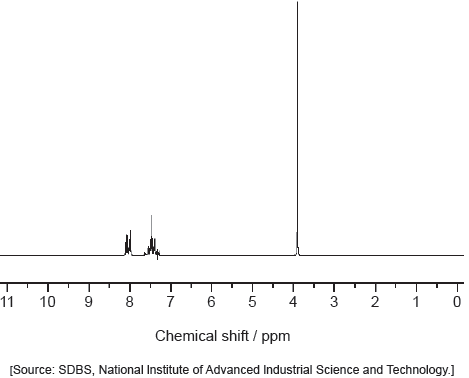
Predict the number of 1H NMR signals, and splitting pattern of the –CH3 seen for propanone (CH3COCH3) and propanal (CH3CH2CHO).
Predict the fragment that is responsible for a m/z of 31 in the mass spectrum of propan‑1‑ol. Use section 28 of the data booklet.
Markscheme
«» 0.2000 «mol of C/CO2»
AND «» 0.2000 «mol of H2O»/0.4000 «mol of H»
OR
«» 2.402 «g of C»
OR
«» 0.404 «g of H» ✔
«4.406 g − 2.806 g» = 1.600 «g of O» ✔
«»
C2H4O ✔
Award [3] for correct final answer.
«» C4H8O2 ✔
C2S2 if CS used.
Award [1 max] for correctly identifying all 3 compounds without valid reasons given.
Accept specific values of wavenumbers within each range.
CH3O+ ✔
Accept any structure i.e. “CH2OH+”.