| Date | November 2015 | Marks available | 4 | Reference code | 15N.2.SL.TZ0.5 |
| Level | Standard level | Paper | Paper 2 | Time zone | Time zone 0 |
| Command term | Deduce | Question number | 5 | Adapted from | N/A |
Question
This question is in two parts. Part 1 is about energy resources. Part 2 is about thermal physics.
Part 1 Energy resources
Electricity can be generated using nuclear fission, by burning fossil fuels or using pump storage hydroelectric schemes.
In a nuclear reactor, outline the purpose of the
Fission of one uranium-235 nucleus releases 203 MeV.
This question is in two parts. Part 1 is about energy resources. Part 2 is about thermal physics.
Part 2 Thermal physics
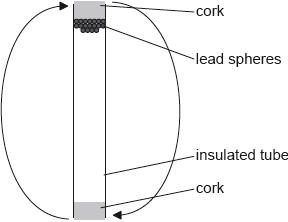
A mass of 0.22 kg of lead spheres is placed in a well-insulated tube. The tube is turned upside down several times so that the spheres fall through an average height of 0.45 m each time the tube is turned. The temperature of the spheres is found to increase by 8 °C.
Outline which of the three generation methods above is renewable.
heat exchanger.
moderator.
Determine the maximum amount of energy, in joule, released by 1.0 g of uranium-235 as a result of fission.
Describe the main principles of the operation of a pump storage hydroelectric scheme.
A hydroelectric scheme has an efficiency of 92%. Water stored in the dam falls through an average height of 57 m. Determine the rate of flow of water, in \({\text{kg}}\,{{\text{s}}^{ - 1}}\), required to generate an electrical output power of 4.5 MW.
Distinguish between specific heat capacity and specific latent heat.
Discuss the changes to the energy of the lead spheres.
The specific heat capacity of lead is \(1.3 \times {10^2}{\text{ J}}\,{\text{k}}{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\). Deduce the number of times that the tube is turned upside down.
Markscheme
pump storage;
renewable as can be replaced in short time scale / storage water can be pumped back up to fall again / source will not run out; } (do not accept “because water is used”)
(allows coolant to) transfer thermal/heat (energy) from the reactor/(nuclear) reaction to the water/steam;
Must see reference to transfer – “cooling reactor/heating up water” is not enough.
reduces speed/kinetic energy of neutrons; (do not allow “particles”)
improves likelihood of fission occurring/U-235 capturing neutrons;
(203 MeV is equivalent to) \(3.25 \times {10^{ - 11}}\) (J);
\(6.02 \times {10^{23}}\) nuclei have a mass of 235 (g) / evaluates number of nuclei;
(\(2.56 \times {10^{21}}\) nuclei produce) \(8.32 \times {10^{10}}\) (J) / multiplies two previous answers;
Award [3] for bald correct answer.
Award [1] for correct conversion from eV to J even if rest is incorrect.
water flows between water masses/reservoirs at different levels;
flow of water drives turbine/generator to produce electricity;
at off peak times the electricity produced is used to raise water from lower to higher reservoir;
use of \(\frac{{mgh}}{t}\);
\(\frac{m}{t} = \frac{{4.5 \times {{10}^6}}}{{0.92 \times 9.81 \times 57}}\); (t is usually ignored, assume 1 s if not seen)
\(8.7 \times {10^3}{\text{ (kg}}\,{{\text{s}}^{ - 1}}{\text{)}}\);
Award [3] for a bald correct answer.
specific heat capacity is/refers to energy required to change the temperature (without changing state);
specific latent heat is energy required to change the state/phase without changing the temperature;
If definitions are given they must include salient points given above.
gravitational potential energy \( \to \) kinetic energy;
kinetic energy \( \to \) internal energy/thermal energy/heat energy;
Do not allow heat.
Two separate energy changes must be explicit.
use of \(mc\Delta T\);
use of \(n \times mg\Delta h\);
equating \((c\Delta T = ng\Delta h)\);
236 or 240;
or
use of \(\Delta U = mc\Delta T\);
\((0.22 \times 1.3 \times {10^2} \times 8 = ){\text{ }}229{\text{ (J)}}\);
\(n \times mg\Delta h = 229{\text{ (J)}}\);
\(n = \frac{{229}}{{0.22 \times 9.81 \times 0.45}} = 236\) or 240; } (allow if answer is rounded up to give complete number of inversions)
Award [4] for a bald correct answer.
Examiners report
The essential difference between specific heat capacity and specific latent heat is that the former refers to a change of temperature without changing state; whereas the latter refers to a change of state without changing temperature. Most candidates just wrote definitions which they had learnt by rote – and omitted the constant temperature for a substance changing state.
This is a question specifically about energy changes so candidates are expected to use accurate language and spell out the changes one by one. Common mistakes were omitting the “gravitational” in gravitational potential energy; referring to “heat” rather than thermal energy; and saying that gravitational potential energy changed to thermal and kinetic energy as if it were a single process.
This was generally well done. There were four marks and the question asks the candidates to “deduce” so it is essential that the argument is transparent. The examiner cannot be expected to search through a mass of numbers in order to carry forward an error.

