| Date | November 2009 | Marks available | 1 | Reference code | 09N.1.SL.TZ0.9 |
| Level | Standard level | Paper | Paper 1 | Time zone | Time zone 0 |
| Command term | Question number | 9 | Adapted from | N/A |
Question
In the table below, which row shows the correct conversion between the Kelvin and Celsius temperature scales?
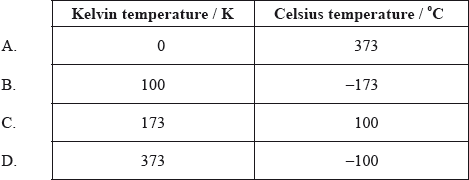
Markscheme
B
Examiners report
[N/A]
Syllabus sections
Show 97 related questions
- 17N.3.SL.TZ0.1b.i: Determine the gradient of the line at a temperature of 80 °C.
- 17N.3.SL.TZ0.1c.ii: Using an appropriate error calculation, justify the number of significant figures that should...
- 17N.3.SL.TZ0.1c.i: Calculate the energy required to raise the temperature of the water from 75 °C to 85 °C.
- 17N.2.SL.TZ0.4b.ii: Outline the difference between the molecular structure of a solid and a liquid.
- 17N.2.SL.TZ0.4b.i: Determine the energy required to melt all of the ice from –20 °C to water at a temperature of...
- 17N.1.SL.TZ0.10: A 1.0 kW heater supplies energy to a liquid of mass 0.50 kg. The temperature of the liquid...
- 17N.1.HL.TZ0.9: The fraction of the internal energy that is due to molecular vibration varies in the...
- 17N.3.SL.TZ0.1b.ii: State the unit for the quantity represented by the gradient in your answer to (b)(i).
- 17M.2.HL.TZ2.3c.ii: Suggest one other energy loss in the experiment and the effect it will have on the value for...
- 17M.2.HL.TZ2.3c.i: The mass of the resistance wire is 0.61 g and its observed temperature rise is 28 K. Estimate...
- 17M.2.HL.TZ1.6d: At the instant of impact the meteorite which is made of ice has a temperature of 0 °C. Assume...
- 17M.2.SL.TZ1.1a.ii: Some of the gravitational potential energy transferred into internal energy of the skis,...
- 17M.1.SL.TZ2.11: A mass m of ice at a temperature of –5 °C is changed into water at a temperature of 50...
- 17M.1.SL.TZ2.10: The graph shows the variation with time t of the temperature T of two samples, X and Y. X and...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the...
- 17M.1.SL.TZ1.10: A liquid is initially at its freezing point. Energy is removed at a uniform rate from the...
- 16N.2.SL.TZ0.3a: Define internal energy.
- 16M.2.SL.TZ0.3b: The experiment is repeated using the same mass of crushed ice. Suggest the effect, if any,...
- 16M.2.SL.TZ0.3a: Using the data, estimate the specific latent heat of fusion of ice.
- 16N.1.SL.TZ0.10: Energy is supplied at a constant rate to a fixed mass of a material. The material begins as a...
- 16M.1.HL.TZ0.7: A container with 0.60kg of a liquid substance is placed on a heater at time t=0. The...
- 16M.1.SL.TZ0.10: A substance is heated at constant power. The graph...
- 15M.1.HL.TZ1.6: Which of the following is numerically equal to the specific heat capacity of the substance of...
- 15M.1.SL.TZ2.8: Which of the following is equivalent to a temperature of –100°C? A. –373 K B. –173 K C....
- 15M.1.SL.TZ2.10: Equal masses of water at 80°C and paraffin at 20°C are mixed in a container of negligible...
- 15M.1.SL.TZ1.10: Molecules leave a boiling liquid to form a vapour. The vapour and the liquid have the same...
- 15M.1.SL.TZ2.9: A sample of solid copper is heated beyond its melting point. The graph shows the variation of...
- 15M.2.SL.TZ1.3a: Explain, in terms of the energy of its molecules, why the temperature of a pure substance...
- 15M.2.SL.TZ2.3b: This question is about internal energy. (i) Mathilde raises the temperature of water in an...
- 14M.1.SL.TZ1.10: A fixed mass of water is heated by an electric heater of unknown power P. The following...
- 14M.1.SL.TZ1.11: A block of iron of mass 10 kg and temperature 10°C is brought into contact with a block of...
- 14M.1.SL.TZ2.11: The specific latent heat is the energy required to change the phase of A. one kilogram of a...
- 14M.1.HL.TZ1.13: An ideal gas expands at constant pressure. The graph shows the relationship between pressure...
- 14M.2.SL.TZ1.5e: (i) Define the specific latent heat of fusion of a substance. (ii) Explain, in terms of the...
- 14M.2.SL.TZ1.5f: A piece of ice is placed into a beaker of water and melts completely. The following data are...
- 15N.1.SL.TZ0.10: When 1800 J of energy is supplied to a mass m of liquid in a container, the temperature of...
- 15N.1.SL.TZ0.8: A container holds 40 g of argon-40...
- 15N.1.SL.TZ0.11: Two objects are in thermal contact and are at different temperatures. What is/are determined...
- 14N.1.SL.TZ0.9: Two objects are in thermal contact, initially at different temperatures. Which of the...
- 14N.1.SL.TZ0.11: The following can be determined for a solid substance. I. The average kinetic...
- 15N.2.SL.TZ0.5f.i: Discuss the changes to the energy of the lead spheres.
- 15N.2.SL.TZ0.5e: Distinguish between specific heat capacity and specific latent heat.
- 15N.2.SL.TZ0.5f.ii: The specific heat capacity of lead is...
- 14N.1.HL.TZ0.6: Two objects are in thermal contact, initially at different temperatures. Which of the...
- 14N.2.SL.TZ0.4e: Describe, with reference to molecular behaviour, the process of melting ice.
- 14N.2.SL.TZ0.4f.ii: The following data are available. Specific heat capacity of water ...
- 14N.2.SL.TZ0.4g: The whole of the experiment in (f)(i) and (f)(ii) is repeated with a container of negligible...
- 14N.2.SL.TZ0.4f.i: After a time interval of 45.0 s all of the ice has reached a temperature of 0 °C without any...
- 14M.2.SL.TZ2.2a: Outline why a given mass of molten zinc has a greater internal energy than the same mass of...
- 14M.2.SL.TZ2.2b: Molten zinc cools in an iron mould. The temperature of the iron mould was 20° C before the...
- 14M.2.SL.TZ2.4a: State the difference between renewable and non-renewable energy sources.
- 11N.1.SL.TZO.10: A pure solid is heated at its melting point. While it is melting the A. mean kinetic energy...
- 11N.1.SL.TZO.11: Which of the following is equivalent to a temperature of 350 K? A. –623°CB. –77°CC. +77°CD....
- 11N.1.SL.TZO.12: A liquid-in-glass thermometer is in thermal equilibrium with some hot water. The thermometer...
- 12N.1.SL.TZ0.12: A mass of 0.20 kg of water at 20°C is mixed with 0.40 kg of water at 80°C. No thermal energy...
- 12N.1.SL.TZ0.14: The internal energy of any substance is made up of the A. total random kinetic and potential...
- 11N.1.HL.TZ0.10: The molar mass of magnesium is 24g. 12g of magnesium contains the same number of particles...
- 12N.1.SL.TZ0.13: What is the temperature, in K, that is equivalent to 57°C? A. 220B. 273C. 330D. 430
- 13N.1.SL.TZ0.9: Molar mass is defined as A. the number of particles in one mole of a substance.B....
- 13N.1.SL.TZ0.11: A solid of mass m is initially at temperature ΔT below its melting point. The solid has...
- 12M.1.SL.TZ2.11: The specific latent heat of a substance is defined as the energy required at constant...
- 12M.1.SL.TZ2.9: Thermal energy is transferred to a solid. Three properties of the solid are I. volumeII....
- 12M.1.SL.TZ1.11: An ideal gas has an absolute temperature T. The average random kinetic energy of the...
- 12M.1.SL.TZ1.9: The total potential energy and random kinetic energy of the molecules of an object is equal...
- 11M.1.SL.TZ2.10: Oil with volume V has specific heat capacity c at temperature T. The density of oil is ρ....
- 12M.1.HL.TZ2.10: Which of the following correctly identifies the properties of the molecules of a substance...
- 13M.2.HL.TZ1.12a: With respect to a gas, explain the meaning of the terms thermal energy and internal energy.
- 13M.2.SL.TZ2.5a: Distinguish between internal energy and thermal energy (heat). Internal energy: Thermal...
- 13M.2.SL.TZ2.5b: A 300 W immersion heater is placed in a beaker containing 0.25 kg of water at a temperature...
- 12M.2.SL.TZ2.4b: Argon behaves as an ideal gas for a large range of temperatures and pressures. One mole of...
- 12M.2.SL.TZ2.4c: At the temperature of 350 K, the piston in (b) is now freed and the argon expands until its...
- 13M.1.SL.TZ2.9: The temperature of an object is -153°C. Its temperature is raised to 273°C. What is the...
- 11M.2.SL.TZ2.3b: Describe, with reference to the energy of the molecules, the difference in...
- 11M.2.SL.TZ2.3a: Distinguish between internal energy and thermal energy.
- 11M.2.SL.TZ2.3c: A piece of iron is placed in a kiln until it reaches the temperature θ of...
- 12M.2.SL.TZ1.3a: Define specific heat capacity.
- 12M.2.SL.TZ1.3b: The following data are available. Mass of water = 0.35 kgMass of iron = 0.58 kgSpecific heat...
- 11N.2.SL.TZ0.5a: Distinguish between the concepts of internal energy and temperature.
- 11N.2.SL.TZ0.5c: An athlete loses 1.8 kg of water from her body through sweating during a training session...
- 11N.2.HL.TZ0.2a: Distinguish between the concepts of internal energy and temperature.
- 12N.2.SL.TZ0.7a: The Pobeda ice island forms regularly when icebergs run aground near the Antarctic ice shelf....
- 12N.2.SL.TZ0.7b: Suggest the likely effect on the average albedo of the region in which the island was...
- 13N.2.SL.TZ0.4h: In an experiment to measure the specific latent heat of vaporization of water, steam at 100°C...
- 13N.2.SL.TZ0.4i: Explain why, other than measurement or calculation error, the accepted value of L is greater...
- 13N.2.SL.TZ0.4g: Water at constant pressure boils at constant temperature. Outline, in terms of the energy of...
- 11M.1.SL.TZ1.11: What is the mass of carbon-12 that contains the same number of atoms as 14 g of...
- 11M.1.SL.TZ1.12: A heater of constant power heats a liquid of mass m and specific heat capacity c. The graph...
- 11M.1.SL.TZ1.10: A solid piece of tungsten melts into liquid without a change in temperature. Which of the...
- 11M.2.SL.TZ1.6c: After 10 s the ball has fallen 190 m. (i) Show that the sum of the potential and kinetic...
- 09M.1.SL.TZ1.9: A temperature of 23 K is equivalent to a temperature of A. \( - 300\) °C. B. ...
- 10M.1.HL.TZ1.10: Water at a temperature of 0 °C is kept in a thermally insulated container. A lump of ice,...
- 10N.1.HL.TZ0.9: An ice cube and an iceberg are both at a temperature of 0 °C. Which of the following is a...
- 09N.1.SL.TZ0.11: Tanya heats 100 g of a liquid with an electric heater which has a constant power output of 60...
- 10N.1.SL.TZ0.9: A system consists of an ice cube placed in a cup of water. The system is thermally insulated...
- 10N.1.SL.TZ0.10: Thermal energy is added at a constant rate to a substance which is solid at time \(t = 0\)....
- 09N.1.SL.TZ0.10: Carbon has a relative atomic mass of 12 and oxygen has a relative atomic mass of 16. A sample...
- 10N.2.SL.TZ0.B2Part2.c: State, in terms of molecular structure and their motion, two differences between a liquid and...

