| Date | May 2009 | Marks available | 1 | Reference code | 09M.1.sl.TZ2.19 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Deduce | Question number | 19 | Adapted from | N/A |
Question
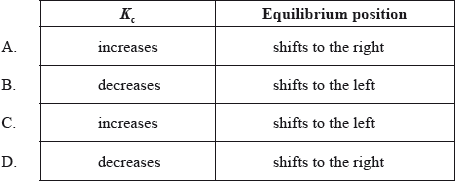
What effect will an increase in temperature have on the \({K_{\text{c}}}\) value and the position of equilibrium in the following reaction?
\[\begin{array}{*{20}{l}} {{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}}&{\Delta H = - 92{\text{ kJ}}} \end{array}\]
Markscheme
B
Examiners report
The difficulty index for this question was 58%, with incorrect responses distributed quite evenly over the distracters. It did however prove to be the best discriminator on the paper with a discrimination index of 0.64.
Syllabus sections
Show 146 related questions
- 17N.2.sl.TZ0.5a: The following reaction was allowed to reach equilibrium at 761 K. H2 (g) + I2 (g)...
- 17N.1.sl.TZ0.18: What will happen if the pressure is increased in the following reaction mixture at...
- 17N.2.hl.TZ0.6a.i: State the equilibrium constant expression, Kc , for this reaction.
- 17N.1.hl.TZ0.19: The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement...
- 17M.3.sl.TZ2.2b.ii: Suggest one improvement to the investigation.
- 17M.3.sl.TZ2.14b.ii: Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to...
- 17M.3.sl.TZ2.1c.i: The equilibrium expression for O2 exchange between the atmosphere and ocean is O2(g)...
- 17M.2.sl.TZ2.3a.ii: Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14...
- 17M.2.sl.TZ2.3a.i: Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
- 17M.1.hl.TZ2.22: Which variable affects the equilibrium constant, Kc? A. Atmospheric pressure B. ...
- 17M.1.sl.TZ2.18: What is the equilibrium constant expression, Kc, for the following reaction? 2NH3(g) +...
- 17M.3.sl.TZ1.20b: Explain the effect of a large amount of aspirin on the pH of blood.
- 17M.3.sl.TZ1.17c: Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide...
- 17M.2.sl.TZ1.4c: Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction...
- 17M.2.sl.TZ1.4b: Ammonia reacts reversibly with water. NH3(g) + H2O(l) \( \rightleftharpoons \) NH4+(aq) +...
- 17M.1.sl.TZ1.18: Consider the equilibrium between N2O4(g) and NO2(g). N2O4(g) \( \rightleftharpoons...
- 16N.3.sl.TZ0.1b: CT values are influenced by temperature and by pH. The table below shows the CT values for...
- 16N.2.sl.TZ0.1a: Ethane-1,2-diol can be formed according to the following reaction. 2CO (g) +...
- 16N.1.sl.TZ0.18: What happens when the temperature of the following equilibrium system is increased? CO(g) +...
- 16M.2.hl.TZ0.4a: (i) State why you would expect tin(II) chloride to have a similar lattice enthalpy to...
- 16M.2.hl.TZ0.2a: (i) Deduce the equilibrium constant expression, Kc, for this reaction. (ii) At exactly 600°C...
- 16M.2.sl.TZ0.3a: (i) Deduce the equilibrium constant expression, Kc, for this reaction. (ii) State the effect...
- 16M.1.sl.TZ0.18: What is the effect of...
- 15M.2.hl.TZ1.3c.i: The Contact process involves this homogeneous...
- 15M.2.hl.TZ1.3c.iii: The Contact process involves this homogeneous...
- 15M.2.hl.TZ1.3d: Outline the economic importance of using a catalyst in the Contact process.
- 15M.2.hl.TZ2.7a.i: Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
- 15M.2.hl.TZ2.7a.ii: Predict, with a reason, how each of the following changes affects the position of...
- 15M.2.hl.TZ2.7c: Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in...
- 15M.2.hl.TZ2.7d.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction on page 10.
- 15M.1.sl.TZ1.20: Carbon monoxide and water react together in the industrial production of hydrogen...
- 15M.1.sl.TZ1.19: Which change will favour the reverse reaction in the...
- 15M.1.sl.TZ2.20: Which combination of temperature and pressure will give the greatest yield of sulfur...
- 15M.2.sl.TZ2.5b: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
- 15M.2.sl.TZ2.5e: Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in...
- 15M.2.sl.TZ1.7d.i: Describe two characteristics of a reaction at equilibrium.
- 15M.2.sl.TZ1.7d.iii: State and explain the effect of a catalyst on the position of equilibrium.
- 15M.2.sl.TZ2.5a: Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
- 15M.2.sl.TZ2.5c: Predict, with a reason, how each of the following changes affects the position of...
- 14M.1.hl.TZ2.8: Which statements explain why a catalyst is used in the Contact process (shown...
- 14M.2.hl.TZ2.1d: Outline how you could establish that the system had reached equilibrium at the end of one week.
- 14M.2.hl.TZ1.7a: (i) Deduce the equilibrium constant expression for this reaction. (ii) Explain...
- 14M.2.hl.TZ2.1c.iv: Deduce the equilibrium constant expression for the reaction.
- 14M.2.hl.TZ2.5b.i: Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is...
- 14M.2.hl.TZ2.1f: Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of...
- 14M.2.hl.TZ2.5b.ii: State a balanced equation for the reaction of chloric(I) acid with water.
- 14M.2.hl.TZ2.5b.iii: Outline, in terms of the equilibrium in aqueous chlorine, why it is dangerous to use an...
- 14M.2.hl.TZ2.5b.iv: Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
- 14M.2.hl.TZ2.8e: Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in...
- 15M.1.sl.TZ2.19: What is the equilibrium constant expression, \({K_{\text{c}}}\), for the formation of...
- 14M.1.sl.TZ1.19: Which statement is correct for a reversible reaction when \({K_{\text{c}}} \gg 1\)? A. ...
- 14M.1.sl.TZ1.20: Consider this reaction at equilibrium. ...
- 14M.1.sl.TZ2.19: What is the equilibrium constant expression, \({K_{\text{c}}}\), for this...
- 14M.2.sl.TZ1.3b: Explain the effect on the position of the equilibrium and on the value of \({K_{\text{c}}}\)...
- 14M.1.sl.TZ2.20: Which is always correct for a reaction at equilibrium?
- 14M.2.sl.TZ2.1f: Deduce the equilibrium constant expression for the reaction.
- 14M.2.sl.TZ2.1g: Outline how you could establish that the system had reached equilibrium at the end of one week.
- 14M.2.sl.TZ2.1i: Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of...
- 14M.2.sl.TZ2.4c.i: Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is...
- 14M.2.sl.TZ2.4c.ii: State a balanced equation for the reaction of chloric(I) acid with water.
- 14M.2.sl.TZ2.4c.iii: Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet...
- 14M.2.sl.TZ2.4c.iv: Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
- 14N.2.hl.TZ0.3c: (i) A sample of liquid bromine was left in a closed conical (Erlenmeyer) flask at 298 K...
- 14N.1.sl.TZ0.19: Which equilibrium reaction shifts to the product side when the temperature is increased at...
- 14N.1.sl.TZ0.20: Which statement correctly describes the effect of a catalyst on the equilibrium...
- 14N.2.sl.TZ0.5a: Deduce the extent of the reaction at 200 °C and 1 atm.
- 14N.2.sl.TZ0.5b: The Contact process operates at a temperature of 450 °C and a pressure of 2 atm as optimum...
- 14N.2.sl.TZ0.5c: An engineer at a Contact process plant hypothesized that using pure oxygen, instead of air,...
- 13N.1.hl.TZ0.23: Which of the following will shift the position of equilibrium to the right in the Haber...
- 13N.2.hl.TZ0.6b.ii: Considering the above equilibrium, predict, giving a reason, how adding more acid would...
- 13N.1.sl.TZ0.20: What happens to the position of equilibrium and the value of \({K_{\text{c}}}\) in the...
- 13N.1.sl.TZ0.19: What is the equilibrium constant expression, \({K_{\text{c}}}\), for the following...
- 13N.2.sl.TZ0.5a.ii: Considering the above equilibrium, predict, giving a reason, how adding more acid would...
- 13M.1.sl.TZ1.20: Which changes occur when the temperature is decreased in the following...
- 13M.1.sl.TZ1.19: The value of the equilibrium constant, \({K_{\text{c}}}\), for a reaction is...
- 13M.2.sl.TZ1.7c.i: Outline the characteristics of a chemical equilibrium.
- 13M.2.sl.TZ1.7c.ii: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for this reaction.
- 13M.2.sl.TZ1.7d.i: Increase in temperature.
- 13M.2.sl.TZ1.7d.ii: Increase in pressure.
- 13M.2.sl.TZ1.7d.iii: Addition of a catalyst.
- 13M.2.hl.TZ2.4a.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the formation of HI(g).
- 13M.1.sl.TZ2.19: Which statement is correct when the system is at equilibrium at 350 °C? A. The...
- 13M.1.sl.TZ2.20: Which statement describes and explains the conditions that favour the formation of hydrogen...
- 12N.2.sl.TZ0.3b: (i) Deduce the equilibrium constant expression, \({K_{\text{c}}}\). (ii) Predict how...
- 12N.1.sl.TZ0.19: Consider the following reaction: \({\text{2A}} \rightleftharpoons {\text{C}}\) ...
- 12N.1.sl.TZ0.20: Iron(III) ions, \({\text{F}}{{\text{e}}^{3 + }}\), react with thiocyanate ions,...
- 12N.2.sl.TZ0.3a: A glass container is half-filled with liquid bromine and then sealed. The system eventually...
- 10N.1.hl.TZ0.22: What is the effect of an increase of temperature on the yield and the equilibrium constant...
- 10N.2.hl.TZ0.6a: (i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and...
- 10N.1.sl.TZ0.20: The formation of nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), from...
- 10N.1.sl.TZ0.19: What is the equilibrium constant expression for the reaction...
- 10N.2.sl.TZ0.6a: (i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and...
- 09N.2.hl.TZ0.6e.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
- 09N.2.hl.TZ0.6e.iii: If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each...
- 09N.2.hl.TZ0.6e.v: Suggest, stating a reason, how the addition of a catalyst at constant pressure and...
- 09N.2.hl.TZ0.6a.iii: 1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at...
- 09N.2.hl.TZ0.6e.iv: If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\),...
- 09N.1.sl.TZ0.20: An increase in temperature increases the amount of chlorine present in the following...
- 09N.1.sl.TZ0.21: What will happen when at a constant temperature, more iodide ions, \({{\text{I}}^ - }\), are...
- 09N.2.sl.TZ0.5a.ii: If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each...
- 09N.2.sl.TZ0.5a.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
- 09N.2.sl.TZ0.5a.iv: Suggest, stating a reason, how the addition of a catalyst at constant pressure and...
- 09N.2.sl.TZ0.5a.iii: If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\),...
- 10M.2.sl.TZ1.5d: (i) A state of equilibrium can exist when a piece of copper metal is placed in a solution...
- 10M.2.hl.TZ2.6f.iii: Outline the economic significance of the use of a catalyst in the Haber process which is an...
- 10M.2.hl.TZ2.7a: (i) State the expression for the ionic product constant of water,...
- 10M.1.sl.TZ2.21: Consider the endothermic reaction...
- 10M.2.sl.TZ2.6b: In carbonated drinks containing dissolved carbon dioxide under high pressure, the following...
- 10M.2.sl.TZ2.6a: (i) State the equilibrium constant expression for the dissociation of water. (ii) ...
- 10M.1.sl.TZ2.20: What is the equilibrium constant expression, \({K_{\text{c}}}\), for the following...
- 09M.1.hl.TZ1.24: Consider the following reversible...
- 09M.2.sl.TZ1.1c.i: State what is meant by the term dynamic equilibrium.
- 09M.2.sl.TZ1.1c.ii: Using the abbreviations [vegetable oil], [methanol], [glycerol] and [biodiesel] deduce the...
- 09M.1.sl.TZ1.21: Consider the following equilibrium...
- 09M.2.sl.TZ1.1c.iii: Suggest a reason why excess methanol is used in this process.
- 09M.2.sl.TZ1.1c.iv: State and explain the effect that the addition of the sodium hydroxide catalyst will have on...
- 09M.1.hl.TZ2.24: Which statement is correct for the equilibrium...
- 09M.2.hl.TZ2.6a.ii: State and explain the effect of increasing the pressure on the yield of sulfur trioxide.
- 09M.2.hl.TZ2.6a.iv: State the effects of a catalyst on the forward and reverse reactions, on the position of...
- 09M.2.sl.TZ2.5a.iv: State and explain the effect of a catalyst on the position of equilibrium.
- 09M.2.sl.TZ2.5a.ii: State and explain the effect of increasing the temperature on the yield of sulfur trioxide.
- 09M.1.sl.TZ2.20: Which statement is always correct for a chemical reaction at equilibrium? A. The rate of...
- 09M.2.sl.TZ2.5a.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
- 09M.2.sl.TZ2.5a.iii: State the effect of a catalyst on the value of \({K_{\text{c}}}\).
- 11M.2.hl.TZ1.8b.i: Describe how an indicator works.
- 11M.2.hl.TZ1.8b.ii: Using Table 16 of the Data Booklet, identify the most appropriate indicator for the titration...
- 11M.1.sl.TZ1.18: Consider the reaction between gaseous iodine and gaseous...
- 11M.1.sl.TZ1.19: The equilibrium between nitrogen dioxide, \({\text{N}}{{\text{O}}_{\text{2}}}\), and...
- 11M.1.sl.TZ1.20: Which statement about chemical equilibria implies they are dynamic? A. The position of...
- 11M.2.sl.TZ1.3b.i: increasing the temperature of the reaction at constant pressure.
- 11M.2.sl.TZ1.3a: State the equilibrium constant expression, \({K_{\text{c}}}\), for the production of methanol.
- 11M.2.sl.TZ1.3c: The conditions used in industry during the production of methanol are a temperature of 450 °C...
- 11M.2.sl.TZ1.3b.ii: increasing the pressure of the reaction at constant temperature.
- 11M.1.sl.TZ2.18: For the following reaction \({K_{\text{c}}} = 1.0 \times {10^{ - 5}}\) at 30...
- 11M.1.sl.TZ2.19: The reaction below represents the Haber process for the industrial production of...
- 11M.2.sl.TZ2.6a.iii: Predict what would happen to the position of equilibrium and the value of \({K_{\text{c}}}\)...
- 11M.2.sl.TZ2.6a.iv: The value of \({K_{\text{c}}}\) at 500 K is 160 and the value of \({K_{\text{c}}}\) at 700 K...
- 11M.2.sl.TZ2.6a.v: The reaction can be catalysed by adding platinum metal. State and explain what effect the...
- 11M.2.sl.TZ2.6a.i: Outline the characteristics of a homogeneous chemical system that is in a state of equilibrium.
- 11M.2.sl.TZ2.6a.ii: Deduce the expression for the equilibrium constant, \({K_{\text{c}}}\).
- 12M.1.sl.TZ2.19: What is the equilibrium constant expression, \({K_{\text{c}}}\), for the following...
- 12M.1.sl.TZ2.20: What happens to the position of equilibrium and the value of \({K_{\text{c}}}\) when the...
- 11N.1.sl.TZ0.20: The following are \({K_{\text{c}}}\) values for a reaction, with the same starting conditions...
- 11N.1.sl.TZ0.19: Which are characteristics of a dynamic equilibrium? I. Amounts of products and reactants...
- 11N.2.sl.TZ0.6a.i: Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
- 11N.2.sl.TZ0.6a.ii: Predict the direction in which the equilibrium will shift when the following changes...

