| Date | May 2014 | Marks available | 2 | Reference code | 14M.2.hl.TZ2.8 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Outline | Question number | 8 | Adapted from | N/A |
Question
Magnesium, a reactive metal found in many common minerals, is also an essential nutrient for both plants and animals.
Successive ionization energies of magnesium are given in the table below.

Magnesium metal is mainly used as a component in lightweight alloys, particularly in combination with aluminium and titanium.
Magnesium is usually produced by the electrolysis of molten magnesium chloride.
Define the term first ionization energy.
(i) Explain why the second ionization energy is greater than the first ionization energy.
(ii) Explain why the third ionization energy is much greater than the second ionization energy.
Although magnesium is usually found as \({\text{M}}{{\text{g}}^{2 + }}\) in its compounds, it is possible to use the Born-Haber cycle to investigate the possibility of \({\text{M}}{{\text{g}}^ + }\) being able to form stable compounds.
Use the ionization energy data from part (b), along with the other data provided below, to determine the enthalpy change of formation of MgCl(s). Assume that, because \({\text{M}}{{\text{g}}^ + }\) would be similar in size to \({\text{N}}{{\text{a}}^ + }\), MgCl would have a similar lattice enthalpy to NaCl.
Enthalpy of atomization of Mg \( + 146{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Bond enthalpy in \({\text{C}}{{\text{l}}_{\text{2}}}\) \( + 243{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Electron affinity of Cl \( + 349{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Lattice enthalpy of NaCl \( + 790{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Consider the lattice enthalpies of \({\text{Mg}}{{\text{F}}_{\text{2}}}\), \({\text{MgC}}{{\text{l}}_2}\) and \({\text{CaC}}{{\text{l}}_{\text{2}}}\). List these from the most endothermic to the least endothermic and explain your order.
\({\text{Most endothermic}} \to {\text{Least endothermic}}\)
Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in water and the equilibrium below exists when excess solid is in contact with a saturated solution.
\[{\text{Mg(OH}}{{\text{)}}_2}{\text{(s)}} \rightleftharpoons {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline how the solubility of magnesium hydroxide will vary with pH.
(i) Describe the bonding present in magnesium metal.
(ii) Suggest why magnesium is harder than sodium.
(iii) Outline why alloys are generally less malleable than their component metals.
(i) Draw a labelled diagram of a suitable apparatus for the electrolysis.
(ii) State equations for the reactions that take place at the electrodes.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iii) When dilute aqueous magnesium chloride is used as the electrolyte, the reactions at both electrodes are different. State equations for the reactions that occur in aqueous solution.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iv) Outline why magnesium metal is not produced in the electrolysis of aqueous magnesium chloride.
Markscheme
minimum energy required to remove one electron / energy required to remove most loosely bound/outermost electron;
from gaseous/isolated atom;
Accept “gaseous state”.
More extensive definitions involving one mole may be given.
(i) electrons lost in same orbital/valence shell;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\) is) closer to the nucleus;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\)) not subject to e-e repulsion from others in same level;
Apply OWTTE for all marking points.
Do not accept “less electrons to share the charge” or answers employing this concept.
(ii) electron in lower energy level / more stable electron shell;
electron closer to nucleus;
less shielding by complete inner shells / increase in effective nuclear charge;
Apply OWTTE for all marking points.
\(\Delta {H_{{\text{at}}}}{\text{(Cl)}} = \frac{1}{2} \times 243{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Correct calculation of atomization enthalpy of Cl.
\(\Delta {H_{\text{f}}} = + 146 + \frac{1}{2}243 + 738 + ( - 349) + ( - 790)\);
Correct sign and magnitude of all terms.
\( = - {\text{134 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Final mark involves correct computation of equation the student has produced.
Award [2] for –12 (bond enthalpy of Cl not halved) or +134 (signs wrong).
Award [1] for +12 (bond enthalpy of Cl not halved and signs wrong).
\({\text{Mg}}{{\text{F}}_2}\) –\({\text{MgC}}{{\text{l}}_2}\) –\({\text{CaC}}{{\text{l}}_2}\);
\({{\text{F}}^ - }\) smaller (ionic radius) than \({\text{C}}{{\text{l}}^ - }\) / \({\text{C}}{{\text{l}}^ - }\) larger (ionic radius) than \({{\text{F}}^ - }\);
\({\text{M}}{{\text{g}}^{2 + }}\) smaller (ionic radius) than \({\text{C}}{{\text{a}}^{2 + }}\) / \({\text{C}}{{\text{a}}^{2 + }}\) larger (ionic radius) than \({\text{M}}{{\text{g}}^{2 + }}\);
Accept use of atomic radius rather than ionic radius.
more soluble at low pH / less soluble at high pH;
higher pH / \({\text{O}}{{\text{H}}^ - }\) will shift the equilibrium to the left / lower pH / \({{\text{H}}^ + }\) will (react with \({\text{O}}{{\text{H}}^ - }\) and) shift the equilibrium to the right;
(i) lattice/layers/framework of cations/magnesium ions/\({\text{M}}{{\text{g}}^{2 + }}\);
surrounded by delocalized electrons / in a sea/flux of delocalized electrons;
Accept “mobile” instead of “delocalized”.
(ii) Mg has more delocalized electrons (than Na);
Accept “Mg has more valence electrons than Na” / “Mg is Mg2+ but Na is only Na+”.
(iii) layers of ions/atoms/particles cannot slide over each other so easily (as different sized ions/atoms/particles) / OWTTE;
(i) 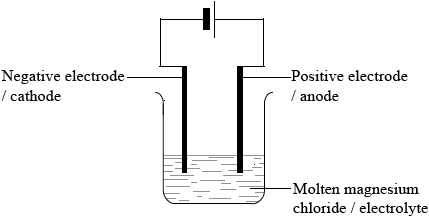
Diagram:
two electrodes connected to a power pack/battery and immersed in an electrolyte;
Do not award mark if salt bridge included in diagram.
Labelling:
anode/positive electrode, cathode/negative electrode, molten magnesium chloride/MgCl2 (l)/electrolyte correctly labelled;
Check candidates know which end of a battery symbol is which charge.
(ii) Negative electrode (cathode): \({\text{M}}{{\text{g}}^{2 + }}{\text{(l)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Mg (s)}}\);
Positive electrode (anode): \[{\text{2C}}{{\text{l}}^ - }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }\];
Accept \(C{l^ - }(l) \to \frac{1}{2}C{l_2}(g) + {e^ - }\).
Ignore state symbols.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}/{\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}}\);
Accept \(4{H_2}O(l) + 4{e^ - } \to 2{H_2}(g) + 4O{H^ - }(aq) / 4{H^ + }(aq) + 4{e^ - } \to 2{H_2}(g)\) / \({H_2}O(l) + {e^ - } \to \frac{1}{2}{H_2}(g) + O{H^ - }(aq)/{H^ + }(aq) + {e^ - } \to \frac{1}{2}{H_2}(g)\).
Positive electrode (anode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - }/{\text{4O}}{{\text{H}}^ - }{\text{(aq)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4}}{{\text{e}}^ - }\);
Accept \({H_2}O(l) \to \frac{1}{2}{O_2}(g) + 2{H^ + }(aq) + 2{e^ - } / 2O{H^ - }(aq) \to \frac{1}{2}{O_2}(g) + {H_2}O(l) + 2{e^ - }\).
State symbols not required.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iv) water/hydrogen ions more easily reduced/better oxidizing agents/have a more positive \({E^\Theta }\) (than magnesium ions);
Accept converse statements for magnesium ions.
Accept “magnesium is very reactive/high in reactivity series” / OWTTE.
Examiners report
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.

