| Date | May 2009 | Marks available | 1 | Reference code | 09M.2.hl.TZ2.3 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | State | Question number | 3 | Adapted from | N/A |
Question
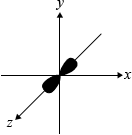
Draw the shape of the \({{\text{p}}_{\text{z}}}\) orbital using the coordinates shown.
State the electron configuration of \({\text{F}}{{\text{e}}^{3 + }}\).
Define the term ligand.
Explain why the complex \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) is coloured.
The element selenium \({\text{(}}Z = 34{\text{)}}\) has electrons in the 4s, 3d and 4p orbitals. Draw an orbital box diagram (arrow-in-box notation) to represent these electrons.
Markscheme
dumbbell-shaped representation along the \(z\)-axis:
 ;
;
Sign of wave function not required.
\({\text{1}}{{\text{s}}^{\text{2}}}2{{\text{s}}^{\text{2}}}2{{\text{p}}^6}{\text{3}}{{\text{s}}^2}{\text{3}}{{\text{p}}^6}{\text{3}}{{\text{d}}^5}/{\text{1}}{{\text{s}}^2}{\text{2}}{{\text{s}}^2}{\text{2}}{{\text{p}}^6}{\text{3}}{{\text{s}}^2}{\text{3}}{{\text{p}}^6}{\text{4}}{{\text{s}}^0}{\text{3}}{{\text{d}}^5}/{\text{[Ar]4}}{{\text{s}}^0}{\text{3}}{{\text{d}}^5}/{\text{[Ar]3}}{{\text{d}}^5}\);
Do not allow 2, 8, 13.
Lewis base / (species/ion/molecule) with lone pair and dative covalent/coordinate bond (to metal) / bonds with metal (ion)/complex ion;
has partially filled d subshell/sublevel/orbitals;
d orbitals are split (into two sets of different energies);
colour due to electron transition between (split) d orbitals;
frequencies of visible light absorbed by electrons moving from lower to higher d levels, colour due to remaining frequencies;
Allow wavelength as well as frequency.
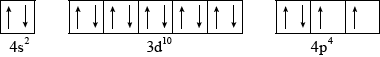 ;
;
Accept half-arrows or full arrows.
Do not penalize if additional sublevels are shown, if sublevels are not labelled or if no boxes are drawn (providing system of arrows correct).
Do not award mark if sublevels are incorrectly labelled.
Orbital diagram may also be represented with sublevels shown at different relative energy positions.
Examiners report
Most candidates were able to draw the dumbbell shaped \({{\text{p}}_{\text{z}}}\) orbital, although some candidates drew all three p-orbitals, hence failing to read the question carefully.
For (ii) the electron configuration of \({\text{F}}{{\text{e}}^{3 + }}\) was well answered compared to recent sessions. The very weak candidates wrote incorrect answers such \({\text{[Ar]4}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{d}}^{\text{2}}}\) and some very weak candidates just gave the electron arrangement 2, 8, 13, which would be a typical SL type answer.
The definition of a ligand was poorly answered (iii) by even the strongest candidates. In general candidates showed some understanding but good definitions were rare. Very often candidates did not mention dative covalent bonding and some said that ligands are simply just lone pairs of electrons.
In part (iv) most candidates referred to the splitting of d orbitals and related colour to d to d transitions. A very high number of candidates scored at least two points. Often candidates did not mention partially filled d subshells or did not score the point: frequencies of visible light absorbed by electrons moving from lower to higher d levels, colour due to remaining frequencies.
The orbital diagram of selenium (v) was very well answered by most candidates. The only minor mistake for the weaker candidates involved lack of understanding of Hund’s Rule for \({\text{4}}{{\text{p}}^{\text{4}}}\).

