| Date | November 2013 | Marks available | 1 | Reference code | 13N.2.hl.TZ0.4 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 4 | Adapted from | N/A |
Question
EUK-134, the structure of which is shown below, is a complex ion of manganese(III) that is used in expensive sun-protection products because of its powerful antioxidant properties.
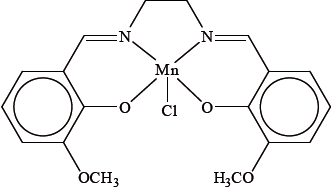
State the electron configuration of the manganese ion in EUK-134.
State the name given to species that bond to a central metal ion, and identify the type of bond present.
Name given:
Type of bond:
Transition metals have certain characteristic properties. State two properties that are involved in EUK-134 rapidly decreasing the concentration of oxidizing agents.
Substances like EUK-134 are often coloured. Explain why compounds of transition metals absorb visible radiation.
Markscheme
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{4}}}\) / \({\text{[Ar]3}}{{\text{d}}^{\text{4}}}\);
ligand;
dative/coordinate (covalent);
Do not accept “covalent”.
variable oxidation state/number;
catalytic properties;
d sublevel/orbitals split (into two levels by ligands);
electrons absorb light/photons and move to the higher energy orbital;
frequency of light/photons absorbed in the visible region;
Examiners report
Very few candidates could interpret the electron structure of manganese from its oxidation state, though the term “ligand” and the nature of its bond to metal ions were almost universally known. The general properties of transition metals seemed to have been well memorized, even though they were not always correctly applied. The splitting of the d sub-shell was generally known, though a worrying number of students believe that transition metal ions emit coloured light.
Very few candidates could interpret the electron structure of manganese from its oxidation state, though the term “ligand” and the nature of its bond to metal ions were almost universally known. The general properties of transition metals seemed to have been well memorized, even though they were not always correctly applied. The splitting of the d sub-shell was generally known, though a worrying number of students believe that transition metal ions emit coloured light.
Very few candidates could interpret the electron structure of manganese from its oxidation state, though the term “ligand” and the nature of its bond to metal ions were almost universally known. The general properties of transition metals seemed to have been well memorized, even though they were not always correctly applied. The splitting of the d sub-shell was generally known, though a worrying number of students believe that transition metal ions emit coloured light.
Very few candidates could interpret the electron structure of manganese from its oxidation state, though the term “ligand” and the nature of its bond to metal ions were almost universally known. The general properties of transition metals seemed to have been well memorized, even though they were not always correctly applied. The splitting of the d sub-shell was generally known, though a worrying number of students believe that transition metal ions emit coloured light.

