| Date | May 2011 | Marks available | 1 | Reference code | 11M.3.sl.TZ2.A1 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | State | Question number | A1 | Adapted from | N/A |
Question
Selected regions of the electromagnetic spectrum are represented in order of increasing frequency below.
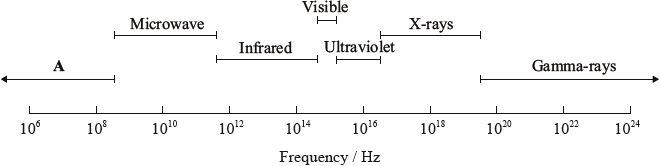
Identify region A.
State which region of the electromagnetic spectrum can be used to identify the functional groups present in a molecule.
Explain why the absorptions in infrared (IR) spectroscopy occur at much higher frequency than those in \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy.
Markscheme
radio(wave);
infrared/IR;
IR involves vibrations of bonds / IR involves shorter wavelength/more energy than \(^{\text{1}}{\text{H}}\,{\text{NMR}}\);
whereas \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) involves transitions between different energy states in the nucleus which are lower in energy / \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) occurs in the radio region therefore energy is lower;
Examiners report
Most candidates were familiar with regions of the electromagnetic spectrum and their uses, but had difficulty describing the relationship between energy and frequency or wavelength.
Most candidates were familiar with regions of the electromagnetic spectrum and their uses, but had difficulty describing the relationship between energy and frequency or wavelength.
Most candidates were familiar with regions of the electromagnetic spectrum and their uses, but had difficulty describing the relationship between energy and frequency or wavelength.

