| Date | November 2014 | Marks available | 3 | Reference code | 14N.2.sl.TZ0.8 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 8 | Adapted from | N/A |
Question
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
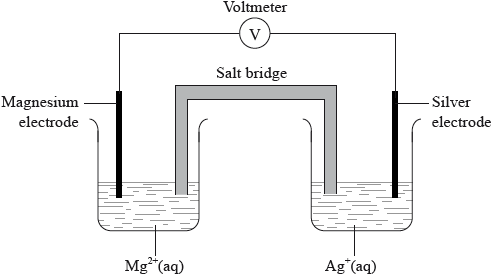
A voltaic cell is made from a half-cell containing a magnesium electrode in a solution of magnesium nitrate and a half-cell containing a silver electrode in a solution of silver(I) nitrate.
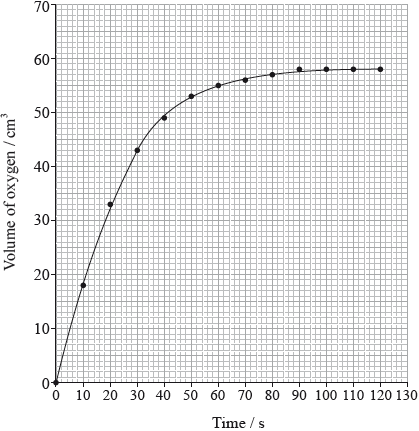
Hydrogen peroxide decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
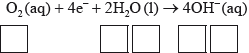
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
(i) Given that magnesium is more reactive than silver, deduce the half-equations for the reactions occurring at each electrode, including state symbols.
Negative electrode (anode):
Positive electrode (cathode):
(ii) Outline one function of the salt bridge.
(i) State the property that determines the order in which elements are arranged in the periodic table.
(ii) State the relationship between the electron arrangement of an element and its group and period in the periodic table.
(i) The experiment is repeated with the same amount of a more effective catalyst, \({\text{Mn}}{{\text{O}}_{\text{2}}}\), under the same conditions and using the same concentration and volume of hydrogen peroxide. On the graph above, sketch the curve you would expect.
(ii) Outline how the initial rate of reaction can be found from the graph.
(iii) Outline a different experimental procedure that can be used to monitor the decomposition rate of hydrogen peroxide.
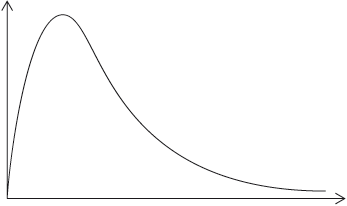
(iv) A Maxwell–Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii) 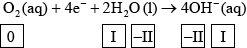 ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
oxygen is non-polar;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
(i) Negative electrode (anode):
\({\text{Mg(s)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }/\frac{1}{2}{\text{Mg(s)}} \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {{\text{e}}^ - }/\)
\({\text{Mg(s)}} - {\text{2}}{{\text{e}}^ - } \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}/\frac{1}{2}{\text{Mg(s)}} - {{\text{e}}^ - } \to \frac{1}{2}{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}}\);
Accept equations for the oxidation of water/hydroxide ions.
Positive electrode (cathode):
\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{Ag (s)}}\);
Accept Ag equation doubled so that both electrodes involve 2 electrons.
Accept e instead of e–.
Award [1 max] if both equations are correct but the state symbols are missing/incorrect.
Award [1 max] if both equations are reversed but state symbols correct.
(ii) provides ions that flow into electrolytes/half-cells / maintains electrical neutrality of solutions/electrolytes / provides electrical continuity by providing path for migrating ions;
Accept completes the (electrical) circuit / allows current to flow / OWTTE.
(i) atomic number / number of protons;
Accept number of electrons in a (neutral) atom.
(ii) groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
(i) steeper curve with a similar shape that reaches same maximum volume of \({{\text{O}}_{\text{2}}}\);
(ii) (draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
(iii) measure/monitor mass/pressure/\({\text{[}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{]}}\);
Accept measure/monitor temperature of system.
(iv) y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.
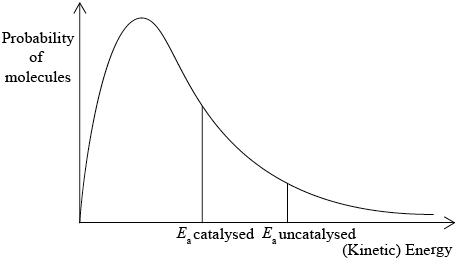
correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by shading and annotating the graph.
Accept a greater number/proportion of successful collisions as catalyst reduces Ea.
Examiners report
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.
In Part (a) almost all candidates could correctly identify the equation as an oxidation reaction and justify their choice. Assigning oxidation numbers to particular species proved slightly trickier, with many not knowing that elements always have an oxidation state of zero.
Combining the half equations also provided a bit of challenge with many equations having residual electrons, though most students could correctly identify the reducing agent. The aqueous solubility of oxygen gas in Part (b) was poorly explained, with the discussion being most frequently in terms of polarity rather than invoking hydrogen bonding. The electrolysis question in Part (c) was generally well answered, though most relied on “completing the circuit” to obtain the salt bridge mark with few showing any comprehension of the way in which this was achieved. Both the property responsible for the ordering of the periodic table and the relationship of electronic structure to position in the periodic table, required for Part (d), were well known and it was rare for a student not to gain full marks. Similarly in Part (e), most students correctly drew the curve that would result from a more effective catalyst. Many also seemed to be aware of the basic idea of how to find the reaction rate, though correct use of the terms “tangent” and “gradient” was rare and many failed to note it referred to “initial rate”. Most students could also identify an appropriate alternative method for monitoring the rate. In the final section most students could accurately label the axes of a Maxwell-Boltzmann curve and many could also use it to explain the effect of a catalyst, though some weaker students confused this with the effect of temperature and constructed a second curve.

