| Date | November 2010 | Marks available | 1 | Reference code | 10N.1.sl.TZ0.15 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Question number | 15 | Adapted from | N/A |
Question
Identical pieces of magnesium are added to two beakers, A and B, containing hydrochloric acid. Both acids have the same initial temperature but their volumes and concentrations differ.
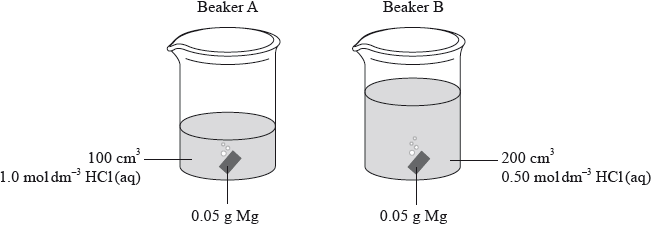
Which statement is correct?
A. The maximum temperature in A will be higher than in B.
B. The maximum temperature in A and B will be equal.
C. It is not possible to predict whether A or B will have the higher maximum temperature.
D. The temperature in A and B will increase at the same rate.
Markscheme
A
Examiners report
One G2 comment stated although this was a good question, it would be challenging for many SL candidates. In fact, although this was the fifth hardest question on the entire paper, 43% of candidates still managed to get the question correct.
Syllabus sections
Show 97 related questions
- 17N.2.sl.TZ0.2b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
- 17N.2.sl.TZ0.1d.ii: Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and...
- 17N.2.sl.TZ0.1d.i: Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid...
- 17N.1.sl.TZ0.13: Which statement is correct for this reaction? Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ...
- 17N.2.hl.TZ0.3b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group...
- 17N.1.hl.TZ0.19: The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement...
- 17N.1.hl.TZ0.17: The combustion of glucose is exothermic and occurs according to the following...
- 17M.2.hl.TZ2.8b.v: State a technique other than a pH titration that can be used to detect the equivalence point.
- 17M.2.sl.TZ2.8a.ii: The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is...
- 17M.3.sl.TZ1.15c: Explain, in terms of the molecular structure, the critical difference in properties...
- 17M.3.sl.TZ1.5f: Outline why the thermochemical method would not be appropriate for 0.001...
- 17M.3.sl.TZ1.5d: State one other assumption that is usually made in the calculation of the heat produced.
- 17M.3.sl.TZ1.5c: Suggest how heat loss could be reduced.
- 17M.3.sl.TZ1.5b: Heat losses would make this method less accurate than the pH probe method. Outline why the...
- 17M.3.sl.TZ1.5a: Explain how the concentration may be calculated in this way.
- 17M.2.sl.TZ1.4f: Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine...
- 17M.2.sl.TZ1.4e: Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages...
- 17M.1.sl.TZ1.13: Which expression gives the mass, in g, of ethanol required to produce 683.5 kJ of heat...
- 16N.1.sl.TZ0.15: 5.35g of solid ammonium chloride, NH4Cl(s), was added to water to form 25.0g of solution. The...
- 16N.1.sl.TZ0.1: Which change of state is exothermic? A. CO2(s) → CO2(g)B. H2O(l) → H2O(g) C. NH3(g) →...
- 16M.3.sl.TZ0.10b: Calculate the energy, in kJ, produced from 15.0g of glucose if its enthalpy of combustion is...
- 16M.2.hl.TZ0.1d: Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and...
- 16M.2.sl.TZ0.2a: (i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol...
- 16M.1.sl.TZ0.13: When 25.0cm3 0.100moldm−3 NaOH(aq) is mixed with 25.0cm3 0.100moldm−3 HCl(aq) at the same...
- 15M.1.hl.TZ1.14: The same amount of heat energy is added to 1.00 g of each substance. Which statement is...
- 15M.1.hl.TZ1.16: Which equation represents the standard enthalpy of formation of liquid methanol? A. ...
- 15M.1.hl.TZ2.16: Which equation represents the standard enthalpy change of formation, \(\Delta H_f^\Theta \),...
- 15M.2.hl.TZ2.3a: Calculate the standard enthalpy change, \(\Delta {H^\Theta }\), in...
- 15M.1.sl.TZ1.14: Which processes are exothermic? I. ...
- 15M.1.sl.TZ2.14: Which combination is correct for the standard enthalpy change of neutralization?
- 15M.2.sl.TZ1.5b.iv: Explain why the reaction is exothermic in terms of the bonds involved.
- 14M.1.sl.TZ1.14: Which statement is correct for the reaction with this enthalpy level diagram? A. Heat...
- 14M.1.sl.TZ1.15: The specific heat capacities of two substances are given in the table below. Which...
- 14M.1.sl.TZ2.14: The table shows information about temperature increases when an acid and an alkali are...
- 14M.1.sl.TZ2.16: What is the temperature rise when 2100 J of energy is supplied to 100 g of water? (Specific...
- 14M.1.sl.TZ2.15: What is the value of \(\Delta H\) for the exothermic reaction represented by the diagram...
- 14M.2.sl.TZ2.6g.iv: The standard enthalpy change of combustion of A is...
- 14M.3.sl.TZ1.5a: When 1.13 g of a granola bar was combusted in a bomb calorimeter, the temperature of...
- 14N.2.hl.TZ0.2b: (i) Define the term standard enthalpy change of formation. (ii) ...
- 14N.2.hl.TZ0.11e: Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and...
- 14N.1.sl.TZ0.14: The enthalpy change for the reaction between zinc metal and copper(II) sulfate solution is...
- 14N.2.sl.TZ0.7e: (i) Define the term standard enthalpy change of reaction, \(\Delta {H^\Theta...
- 13N.1.hl.TZ0.15: Which processes are exothermic? I. ...
- 13N.1.hl.TZ0.17: Which ionic compound has the most endothermic lattice enthalpy? A. Sodium chloride B. ...
- 13N.2.sl.TZ0.4g.ii: Explain how the results of this experiment could be used to calculate the molar enthalpy...
- 13N.1.sl.TZ0.15: Which processes are exothermic? I. ...
- 13N.2.sl.TZ0.4g.i: Methanol can also be burnt as a fuel. Describe an experiment that would allow the molar...
- 13M.1.hl.TZ1.15: Which process is endothermic? A. ...
- 13M.2.sl.TZ1.7a.i: Calculate the enthalpy change of combustion of methanol.
- 13M.2.sl.TZ1.7a.ii: Using the theoretical value in Table 12 of the Data Booklet, discuss the experimental...
- 13M.1.hl.TZ2.16: Which reaction has an enthalpy change equal to the standard enthalpy change of...
- 13M.2.hl.TZ2.5a.iii: Define the term standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \).
- 13M.2.hl.TZ2.5a.iv: Calculate the standard enthalpy change for the reaction, \(\Delta {H^\Theta }\), in...
- 13M.1.sl.TZ2.15: Which statements are correct for an exothermic reaction? I. The products are more stable...
- 13M.1.sl.TZ2.16: The specific heat capacity of aluminium is...
- 13M.2.sl.TZ2.6c.iii: State whether the reaction given in stage 1 is exothermic or endothermic.
- 12N.1.sl.TZ0.14: Which combination is correct for the exothermic reaction that occurs between zinc and copper...
- 12N.1.sl.TZ0.15: A 5.00 g sample of a substance was heated from 25.0 °C to 35.0 °C using...
- 12N.2.sl.TZ0.1a: (i) State the number of significant figures for the masses of...
- 10N.2.hl.TZ0.7c: (i) The enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of liquid...
- 10N.1.sl.TZ0.14: Which statement is correct given the enthalpy level diagram below? A. The reaction is...
- 09N.2.hl.TZ0.7b.i: The standard enthalpy change of three combustion reactions is given below in...
- 09N.2.hl.TZ0.7b.v: Suggest with a reason, why the values obtained in parts (b) (i) and (b) (iv) are different.
- 09N.1.sl.TZ0.16: In a reaction that occurs in 50 g of aqueous solution, the temperature of the reaction...
- 09N.1.sl.TZ0.15: Which is true for a chemical reaction in which the products have a higher enthalpy than the...
- 10M.2.sl.TZ1.6a: (i) Use the data to calculate the heat evolved when the ethanol was combusted. (ii) ...
- 10M.1.sl.TZ2.16: Which is correct about energy changes during bond breaking and bond formation?
- 10M.1.sl.TZ2.17: Which processes are exothermic? I. Ice melting II. Neutralization III. ...
- 09M.1.hl.TZ1.14: 1.0 g of sodium hydroxide, NaOH, was added to 99.0 g of water. The temperature of the...
- 09M.2.hl.TZ1.6b.ii: Deduce which two of the enthalpy changes a to e have negative signs.
- 09M.1.sl.TZ1.15: When some solid barium hydroxide and solid ammonium thiosulfate were reacted together, the...
- 09M.1.sl.TZ1.17: Some water is heated using the heat produced by the combustion of magnesium metal. Which...
- 09M.1.sl.TZ2.14: What is the energy, in kJ, released when 1.00 mol of carbon monoxide is burned according to...
- 09M.2.sl.TZ2.6a.iii: The standard enthalpy change for the complete combustion of octane,...
- 09M.1.sl.TZ2.16: Which of the following reactions are exothermic? I. ...
- 09M.1.sl.TZ2.15: The specific heat of iron is \({\text{0.450 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}}\)....
- 11M.1.sl.TZ1.14: When \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of...
- 11M.2.sl.TZ1.1b.ii: Calculate the heat absorbed, in kJ, by the water.
- 11M.2.sl.TZ1.1c.ii: Part (b)
- 11M.2.sl.TZ1.4a.ii: Calculate the amount, in mol, of ethanol and octane in 1.00 kg of the fuel mixture.
- 11M.2.sl.TZ1.4a.iii: Calculate the total amount of energy, in kJ, released when 1.00 kg of the fuel mixture is...
- 11M.2.sl.TZ1.1b.iii: Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the...
- 11M.2.hl.TZ2.6b.i: Outline why the value for the standard enthalpy change of formation of hydrogen is zero.
- 11M.2.hl.TZ2.6b.ii: Calculate the standard enthalpy change for the hydrogenation of propene.
- 11M.1.sl.TZ2.15: Which processes have a negative enthalpy change? I. ...
- 11M.2.sl.TZ2.1a.iv: Determine the value of \(\Delta {H_1}{\text{ in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
- 11M.1.sl.TZ2.17: At 25 °C, \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of...
- 11M.2.sl.TZ2.1a.ii: Determine what the temperature rise would have been, in °C, if no heat had been lost to the...
- 11M.2.sl.TZ2.1a.iii: Calculate the heat change, in kJ, when 3.99 g of anhydrous copper(II) sulfate is dissolved in...
- 11M.2.sl.TZ2.1b.ii: Determine the value of \(\Delta {H_2}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
- 11M.2.sl.TZ2.6a.iv: The value of \({K_{\text{c}}}\) at 500 K is 160 and the value of \({K_{\text{c}}}\) at 700 K...
- 12M.1.sl.TZ2.15: A simple calorimeter was set up to determine the enthalpy change occurring when one mole of...
- 12M.2.sl.TZ2.6d: (i) Sketch and label an enthalpy level diagram for this reaction. (ii) Deduce...
- 11N.2.hl.TZ0.5b.i: Suggest why the \(\Delta H_{\text{f}}^\Theta \) values for...
- 11N.2.hl.TZ0.5b.ii: Determine the standard enthalpy change at 298 K for the reaction.
- 11N.1.sl.TZ0.15: Which process is endothermic? A. ...
- 11N.2.sl.TZ0.6d.i: Define the term endothermic reaction.

