| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.sl.TZ2.6 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | State | Question number | 6 | Adapted from | N/A |
Question
Chlorine occurs in Group 7, the halogens.
Two stable isotopes of chlorine are \(^{{\text{35}}}{\text{Cl}}\) and \(^{{\text{37}}}{\text{Cl}}\) with mass numbers 35 and 37 respectively.
Chlorine has an electronegativity value of 3.2 on the Pauling scale.
Chloroethene, H2C=CHCl, the monomer used in the polymerization reaction in the manufacture of the polymer poly(chloroethene), PVC, can be synthesized in the following two-stage reaction pathway.
\[\begin{array}{*{20}{l}} {{\text{Stage 1:}}}&{{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}}} \\ {{\text{Stage 2:}}}&{{\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}} + {\text{HC=CHCl(g)}} + {\text{HCl(g)}}} \end{array}\]
Define the term isotopes of an element.
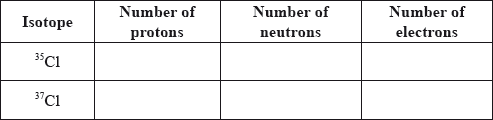
Calculate the number of protons, neutrons and electrons in the isotopes 35Cl and 37Cl.
Using the mass numbers of the two isotopes and the relative atomic mass of chlorine from Table 5 of the Data Booklet, determine the percentage abundance of each isotope.
Percentage abundance 35Cl:
Percentage abundance 37Cl:
Define the term electronegativity.
Using Table 7 of the Data Booklet, explain the trends in electronegativity values of the Group 7 elements from F to I.
State the balanced chemical equation for the reaction of potassium bromide, KBr(aq), with chlorine, Cl2(aq).
Describe the colour change likely to be observed in this reaction.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for stage 1 using average bond enthalpy data from Table 10 of the Data Booklet.
State whether the reaction given in stage 1 is exothermic or endothermic.
Draw the structure of poly(chloroethene) showing two repeating units.
Suggest why monomers are often gases or volatile liquids whereas polymers are solids.
Markscheme
atoms of same element / atoms with same number of protons/atomic number/Z;
Do not allow elements instead of atoms in second alternative.
(but) different numbers of neutrons/mass number/A;
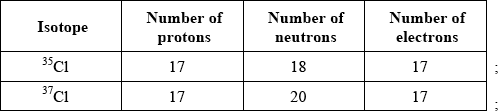
Allow [1 max] for 17 p, 17 e for both if n’s are omitted or incorrect.
Allow [1 max] for 35Cl: 18 n and 37Cl: 20 n if p’s and e’s are omitted.
\(({\text{for}}{{\text{ }}^{{\text{35}}}}{\text{Cl}}:x\% ){\text{ }}35x + 3700 - 37x = 3545\);
Allow other alternative mathematical arrangements.
\(^{{\text{35}}}{\text{Cl}} = 77.5\% \) and \(^{{\text{37}}}{\text{Cl}} = 22.5\% \);
Award [1 max] for correct percentages if no correct working is shown.
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons / OWTTE;
Do not allow element instead of atom/nucleus.
increasing atomic radii (down the group) / OWTTE;
so reduced attraction (for the bonding electrons) / OWTTE;
screening/shielding effect of inner electrons / OWTTE;
Allow more energy levels/electron shells for M1.
Do not accept decrease in nuclear charge.
\({\text{2KBr(aq)}} + {\text{C}}{{\text{l}}_2}{\text{(aq)}} \to {\text{2KCl(aq)}} + {\text{B}}{{\text{r}}_2}{\text{(aq)}}\);
Ignore state symbols.
Allow ionic equation.
colourless/pale yellow/green to yellow/orange/brown;
Start and end colours must both be mentioned.
Bonds breaking:
1 \( \times \) (C=C) \( + \) 4 \( \times \) (C–H) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (4)(413) + (1)(243)/ = ( + )2507{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 4 \( \times \) (C–H) \( + \) 2 \( \times \) (Cl–Cl)
\( = (1)(347) + (4)(413) + (2)(346)/ = - 2691{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((2507 - 2691 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
OR
Bonds breaking:
1 \( \times \) (C=C) \( + \) 1 \( \times \) (Cl–Cl)
\( = (1)(612) + (1)(243)/ = ( + )855{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Bonds forming:
1 \( \times \) (C–C) \( + \) 2 \( \times \) (C–Cl)
\( = (1)(347) + (2)(346)/ = - 1039{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Enthalpy change:
\((855 - 1039 = ){\text{ }} - 184{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
exothermic;
Do not award mark unless based on some value for part (iii).
representation of PVC showing two repeating units;
For example,
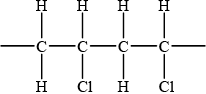
Brackets not necessary but continuation bonds must be given.
No penalty if chlorines are not on same side.
No penalty if chlorines are on two middle C atoms or on two end C atoms.
monomers are smaller molecules / monomers have smaller mass / smaller surface area than polymers;
weaker/fewer intermolecular/London/dispersion/van der Waals’ forces (of attraction);
Allow reverse argument.
Allow abbreviation for London/dispersion as FDL or for van der Waals’ as vdW.
Award zero if reference is made to breaking of bonds.
Examiners report
This was by far the most popular choice of question in Section B. Again, part a) (i) proved challenging as many candidates failed to refer to atoms in their definition and scored only 1 mark out of 2.
In a) (ii) most candidates could state the numbers of protons, neutrons and electrons in the isotopes of chlorine. Those who got this wrong gave answers which indicated a complete lack of understanding of atomic structure.
In a) (iii) some candidates remembered the percentage abundance of chlorine isotopes but could not do the calculation.
Part b) (i) required another definition. Again, many candidates lost marks for inarticulate responses.
The explanation in b) (ii) of trends in electronegativity values was reasonably well done, with most candidates scoring at least one mark out of two.
However, writing a balanced equation in b) (iii) was poorly done with many candidates not knowing the formula of KCl, and not knowing what products would be formed. This is clearly on the syllabus in 3.3.1.
Almost no-one knew the colours of aqueous chlorine and aqueous bromine in b) (iv).
In part c) (ii) the calculation of \(\Delta H\) using bond enthalpies was done well. Some candidates failed to use the C=C bond enthalpy value and some did not recall that bond breaking is endothermic and bond formation exothermic.
Nearly everyone scored a mark in c) (iii) as follow-through marks were awarded.
Drawing two repeating units of poly(chloroethene) presented difficulties in c) (iv). Some candidates tried to draw the monomers joined through the chlorine atoms.
In c) (v) most candidates scored at least one out of two for explaining why monomers have a much lower melting point than polymers.

