| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.hl.TZ2.6 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Calculate | Question number | 6 | Adapted from | N/A |
Question
Acid–base chemistry can play a major role in chemical and biological processes.
White vinegar, which contains ethanoic acid, CH3COOH, can be used as a cleaning agent to dissolve mineral deposits from coffee machines.
Buffer solutions play a pivotal role in solution chemistry.
Acid–base indicators are often organic dyes.
Ammonia, NH3, can be used to clean ovens. The concentration of hydroxide ions, OH–(aq), in a solution of ammonia is \({\text{3.98}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Calculate its pH, correct to one decimal place, at 298 K.
Define an acid according to the Brønsted–Lowry theory and the Lewis theory.
Brønsted–Lowry theory:
Lewis theory:
Ethanoic acid is an example of a weak acid. Distinguish between a strong acid and a weak acid in terms of the extent of dissociation.
State whether the following mixtures, in the appropriate molar ratios, can be classified as buffer solutions. Show your answer by stating yes or no in the table below.
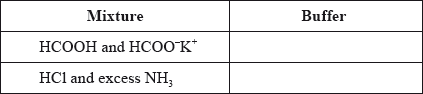
Describe qualitatively the action of an acid–base indicator.
Using Table 16 of the Data Booklet, identify the most appropriate indicator for the titration of ethanoic acid with sodium hydroxide. Explain your choice.
\({\text{150 c}}{{\text{m}}^{\text{3}}}\) of \({\text{5.00}} \times {\text{1}}{{\text{0}}^{ - 1}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) HCl (aq) is mixed with \({\text{300 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.03}} \times {\text{1}}{{\text{0}}^{ - 1}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH(aq). Determine the pH of the solution, correct to two decimal places.
Markscheme
\([{{\text{H}}_3}{{\text{O}}^ + }] = \frac{{{K_{\text{w}}}}}{{[{\text{O}}{{\text{H}}^ - }]}} = \frac{{(1.00 \times {{10}^{ - 14}})}}{{(3.98 \times {{10}^{ - 3}})}} = 2.51 \times {10^{ - 12}}{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
\({\text{pH}}\left( { = - \log [{{\text{H}}_3}{{\text{O}}^ + }] = - \log (2.51 \times {{10}^{ - 12}})} \right) = 11.6\);
OR
\({\text{pOH}} = \left( { - \log (3.98 \times {{10}^{ - 3}}) = } \right){\text{ }}2.4\);
\({\text{pH}} = (14.00 - 2.40) = 11.6\);
Award [2] for correct final answer.
Allow correct use of H+ instead of \({H_3}{O^ + }\) throughout.
Brønsted-Lowry theory:
proton/\({{\text{H}}^ + }\) donor;
Lewis theory:
electron pair acceptor;
Strong acid: acid/electrolyte (assumed to be almost) completely/100% dissociated/ionized (in solution/water) / OWTTE and Weak acid:
acid/electrolyte partially dissociated/ionized (in solution/water) / OWTTE;
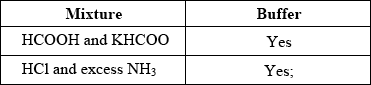
Award [1] for both “yes”.
Award [0] for any “no”.
\(\begin{array}{*{20}{l}} {{\text{HIn(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{I}}{{\text{n}}^ - }{\text{(aq) /}}}&{{\text{HIn(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{I}}{{\text{n}}^ - }{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}} \\ {{\text{Colour}}\,{\text{A Colour}}\,{\text{B}}}&{{\text{Colour}}\,{\text{A Colour}}\,{\text{B}}} \end{array}\);
Allow statement such as solution of weak acid with different colours for conjugate base/In\(^ - \)(aq) and undissociated acid/HIn(aq) / OWTTE.
Equilibrium sign required.
Ignore state symbols.
Allow corresponding argument for an indicator as a weak base.
for example, BOH(aq) \( \to \) B\(^ + \)(aq) + OH\(^ - \)(aq) etc.
in acid/presence of \({{\text{H}}^ + }\) equilibrium lies to left (so colour A);
in alkali/base/presence of \({\text{O}}{{\text{H}}^ - }\) equilibrium lies to right (so colour B);
colour changes/end point when \({\text{[HIn(aq)]}} \approx {\text{[I}}{{\text{n}}^ - }{\text{(aq)]}}\);
phenolphthalein/phenol red;
indicator changes colour in range of pH at equivalence point which is above 7 / OWTTE;
M2 can be scored independently even if indicator is incorrect.
Accept it is a titration of weak acid with a strong base for M2.
\(n{\text{(HCl) }}\left( { = \frac{{(150 \times 5.00 \times {{10}^{ - 1}})}}{{(1000)}}} \right) = 7.50 \times {10^{ - 2}}{\text{ (mol)}}\) and
\(n{\text{(NaOH) }}\left( {\frac{{(300 \times 2.03 \times {{10}^{ - 1}})}}{{(1000)}}} \right) = 6.09 \times {10^{ - 2}}{\text{ (mol)}}\);
\(n{{\text{(HCl)}}_{{\text{remaining}}}}{\text{ }}\left( { = (7.50 - 6.09) \times {{10}^{ - 2}}} \right) = 1.41 \times {10^{ - 2}}{\text{ (mol)}}\);
\({\text{[HCl]}} = (1.41 \times {10^{ - 2}})(1000)/(450) = 3.13 \times {10^{ - 2}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ )}}\);
pH =1.50;
Award [4] for correct final answer.
Award [3 max] for pH = –log (1.41 \( \times \) 10−2 ) =1.85 .
Examiners report
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.
Most candidates calculated the pH of ammonia solution correctly and also the pH of the buffer solution in part (c) (ii). Most students could explain why a solution of the chromium complex is coloured. The difficult part in this question for many was to state and explain whether the salts in solution were acidic, basic or neutral. (e) again caused difficulties for candidates, similar to previous sessions, though many scored some marks for stating acidic. (ii) was very poorly done and M2 was effectively a dead mark.

