| Date | May 2013 | Marks available | 1 | Reference code | 13M.1.hl.TZ1.26 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Deduce | Question number | 26 | Adapted from | N/A |
Question
The values of \({K_{\text{w}}}\), the ionic product constant of water, are:
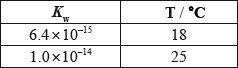
Which statements are correct?
I. The \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) in water is less than the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) at 18 °C.
II. The ionization of water is an endothermic process.
III. The pH of water is lower at 25 °C than at 18 °C.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
[N/A]
Syllabus sections
Additional higher level (AHL) » Topic 18: Acids and bases » 18.2 Calculations involving acids and bases
Show 54 related questions

