| Date | November 2014 | Marks available | 6 | Reference code | 14N.2.hl.TZ0.10 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce, Determine, and State | Question number | 10 | Adapted from | N/A |
Question
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
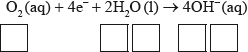
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
State the relationship between the electron arrangement of an element and its group and period in the periodic table.
Transition metals and their compounds often catalyse reactions. The catalyzed decomposition of hydrogen peroxide by CuO is an example. State two other examples of catalyzed reactions giving the transition metal or its compound acting as catalyst.
(i) State a chemical equation for the partial dissociation of water into ions, including state symbols.
(ii) The dissociation of water into ions is reversible. State the expression for the ionic product constant of water.
(iii) The ionic product constant of water was measured at three different temperatures.
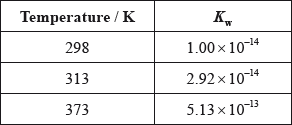
Deduce whether the ionization of water is exothermic or endothermic, giving your reason.
(iv) Use the data in part (iii) to determine the pH of water at 373 K, correct to two decimal places.
(i) An aqueous solution of sodium chloride is electrolysed using inert electrodes. Explain which product is obtained at the positive electrode (anode) if the concentration of sodium chloride is high.
(ii) State the half-equations occurring at the electrodes during the electrolysis of the concentrated aqueous solution of sodium chloride.
Negative electrode (cathode):
Positive electrode (anode):
Describe how electrolysis can be used to electroplate a bracelet with a layer of silver metal. Include the choice of electrodes and electrolyte needed in your description.
Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii) 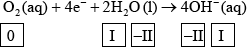 ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
\({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) catalyses oxidation of \({\text{S}}{{\text{O}}_{\text{2}}}\) / \({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) is a catalyst in the Contact Process;
Fe catalyses the reaction between \({{\text{N}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}\) / Fe is a catalyst in the Haber Process;
Ni/Pd/Pt catalyses hydrogenation / manufacture of margarine / addition of hydrogen to C=C / conversion of alkenes to alkanes;
Pd/Pt is a catalyst in catalytic converters / Pd/Pt catalyzes reaction of \({\text{N}}{{\text{O}}_{\text{2}}}\) and CO/\({\text{N}}{{\text{O}}_{\text{2}}}\) and (unburnt) fuel/exhaust gases;
Accept other correct examples.
Accept formulas or names of substances.
(i) \({{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\);
\( \rightleftharpoons \) and state symbols are necessary for the mark.
(ii) \({K_w} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}/{K_w} = {\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}\);
(iii) at higher temperatures ionization increases / at higher temperatures equilibrium shifts to right;
ionization is endothermic;
Do not allow ECF for M2.
(iv) \({\text{5.13}} \times {\text{1}}{{\text{0}}^{ - 13}} = {{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}^2}/{{\text{[}}{{\text{H}}^ + }{\text{]}}^2}/{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = {\text{7.16}} \times {\text{1}}{{\text{0}}^{ - 7}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH}} = 6.14/6.15\);
Award [2] for correct final answer.
(i) chlorine/\({\text{C}}{{\text{l}}_{\text{2}}}\) (is produced at the positive electrode/anode);
according to electrochemical series/ \(E^\circ \) values/ease of oxidation \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released / OWTTE / at low chloride concentration \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released;
high concentration makes \({\text{C}}{{\text{l}}^ - }\) oxidize/react in preference to \({\text{O}}{{\text{H}}^ - }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) / OWTTE;
(ii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}}/{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\);
Positive electrode (anode):
\({\text{2C}}{{\text{l}}^ - }{\text{(aq)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - }{\text{(aq)}} \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{e}}^ - }/{\text{2C}}{{\text{l}}^ - }{\text{(aq)}} - {\text{2}}{{\text{e}}^ - } \to {\text{C}}{{\text{l}}_2}{\text{(g)}}/\)
\({\text{C}}{{\text{l}}^ - }{\text{(aq)}} - {{\text{e}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\);
Ignore state symbols.
Accept e instead of e–.
Award [1] if half-equations are correct but placed at the wrong electrodes.
bracelet/object to be electroplated is the cathode/negative electrode;
silver anode/positive electrode;
Accept Pt anode.
Electrolyte: liquid \({\text{Na[Ag(C}}{{\text{N}}_{\text{2}}}{\text{)]}}\)/sodium dicyanoargentate/\({{\text{[Ag(CN}}{{\text{)}}_{\text{2}}}{\text{]}}^ - }\)/ solution of an appropriate silver salt;
Accept AgNO3/silver nitrate.
All marks can be scored with a labelled diagram.
Examiners report
(i) Very well answered.
(ii) Most candidates answered correctly. The most common mistakes were doubling the oxidation number of H in \({{\text{H}}_{\text{2}}}{\text{O}}\), and entering a wrong oxidation number for elemental oxygen.
(iii) A well-answered question.
The aqueous solubility of oxygen gas was often poorly explained, with the discussion focussing on the intermolecular forces found in each substance separately and then stating that “like dissolves like”.
Well answered by most candidates.
The majority of candidates were able to give two valid examples of transition metals or their compounds acting as catalysts.
(i) Very well answered.
(ii) Well answered.
(iii) About half of the candidates were able to gain full marks. Some candidates found difficulty in connecting the increase in \({K_{\text{w}}}\) to the position of equilibrium.
(iv) About half of the candidates were able to calculate the pH from the \({K_{\text{w}}}\) value.
(i) Many candidates identified chlorine as the product, but the other two marks were more discriminating. Some candidates clarified that \({\text{C}}{{\text{l}}^ - }\) was oxidized in preference to OH- because of its high concentration, but very few related the situation to the electrochemical series.
(ii) This was poorly answered by many candidates. Common mistakes included releasing sodium at the cathode, reversing electrodes and unbalanced redox half-reactions where the electrons were sometimes on the wrong side of the equation.
Very well answered. Most candidates determined both electrodes correctly. The main difficulty for some candidates was choosing a suitable electrolyte.

