| Date | November 2014 | Marks available | 2 | Reference code | 14N.2.hl.TZ0.1 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Determine | Question number | 1 | Adapted from | N/A |
Question
A student used a pH meter to measure the pH of different samples of water at 298 K.
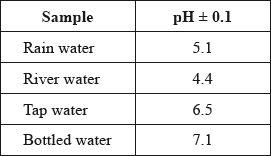
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
Determine the concentration of hydroxide ions in the sample of river water.
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
Markscheme
river (water);
\(\left( {\frac{{0.1}}{{5.1}} \times 100 = } \right){\text{ }}2\% \);
recognition that values differ by 2 pH units / calculation of both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values;
\(({\text{ratio}} = ){\text{ }}1:100/\frac{1}{{100}}/{10^{ - 2}}/0.01\);
Award [2] for correct final answer.
Award [1 max] for 100:1/100/102.
\({\text{pOH}} = (14.0 - 4.4 = ){\text{ }}9.6/{\text{[}}{{\text{H}}^ + }{\text{]}} = 4 \times {10^{ - 5}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
Accept [H+] = 3.98 \( \times \) 10–5 (mol dm–3).
\({\text{[O}}{{\text{H}}^ - }{\text{]}} = 3 \times {10^{ - 10}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
Accept 2.51 \( \times \) 10–10 (mol dm–3).
Award [2] for correct final answer.
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}^ + }/{\text{C}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}_2}{{\text{O}}^ + }/{\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{H}}_2}{\text{C}}{{\text{O}}_3}\);
Do not penalize missing reversible arrow.
Do not accept equations with the carbonate ion as a product.
Examiners report
A very well answered question.
The majority of candidates calculated the percentage uncertainty correctly, however, more than half of them did not pay attention to stating the answer to the appropriate number of significant figures. Some candidates used river water data instead of rain water.
More than half of the candidates calculated the correct ratio of hydrogen ion concentration. The majority of these candidates calculated the concentration of hydrogen ions in both samples, instead of simply using the difference of 2 pH units.
Generally well answered. Some students only scored one mark, stopping at the calculation of the pOH or the concentration of hydrogen.
About half the candidates wrote correct products, however, most of the candidates did not use reversible arrows. Several variations of incorrect products were given including \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) and CO.

