| Date | May 2014 | Marks available | 1 | Reference code | 14M.1.hl.TZ1.26 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Question number | 26 | Adapted from | N/A |
Question
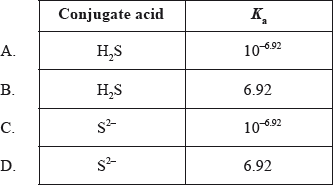
The \({\text{p}}{K_{\text{b}}}\) of \({\text{H}}{{\text{S}}^ - }\) is 7.08. What is its conjugate acid and what is the \({K_{\text{a}}}\) value of the acid?
Markscheme
A
Examiners report
[N/A]
Syllabus sections
Additional higher level (AHL) » Topic 18: Acids and bases » 18.2 Calculations involving acids and bases
Show 54 related questions
- 17N.2.hl.TZ0.6c.ii: Calculate Kb for HCO3– acting as a base.
- 17N.2.hl.TZ0.6c.i: Calculate [H3O+] in the solution and the dissociation constant, Ka , of the acid at 25 °C.
- 17M.2.hl.TZ2.8c: The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two...
- 17M.1.hl.TZ2.27: What is the order of increasing acidity of the following acids? A. chloroethanoic <...
- 17M.2.hl.TZ1.5d.i: Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of...
- 16N.2.hl.TZ0.7a: Calculate the pH of 0.0100 mol dm–3 methanoic acid stating any assumption you make. Ka = 1.6...
- 16M.2.hl.TZ0.2d: Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of...
- 15M.1.hl.TZ1.26: The forward reaction of this equilibrium is...
- 15M.1.hl.TZ1.27: Which equation represents a reaction for which a base dissociation constant expression,...
- 15M.1.hl.TZ2.26: The strengths of four acids are: glycine ...
- 15M.2.hl.TZ1.8b: Calculate the pH, using table 15 of the data booklet, of a solution of ethanoic acid made by...
- 14M.1.hl.TZ2.29: What is the expression for the ionic product constant of water, \({K_{\text{w}}}\)? A. ...
- 14M.2.hl.TZ1.7c.iv: A \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia is added to...
- 14M.2.hl.TZ1.7c.v: Calculate the pH of the solution at the equivalence point, using Table 15 of the Data Booklet.
- 14M.2.hl.TZ2.6e.ii: Sulfur dioxide, a major cause of acid rain, is quite soluble in water and the equilibrium...
- 14M.3.hl.TZ1.24b: The \({\text{p}}{K_{\text{b}}}\) values at 298 K for diethylamine and triethylamine are given...
- 14N.1.hl.TZ0.26: Which compound will produce an aqueous solution which has a pH greater than 7? A. ...
- 14N.1.hl.TZ0.27: Methylamine acts as a weak base when it reacts with water. For a diluted aqueous solution,...
- 14N.2.hl.TZ0.1d: Determine the concentration of hydroxide ions in the sample of river water.
- 14N.2.hl.TZ0.4c: Phosphoric(V) acid, \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\), has a...
- 14N.2.hl.TZ0.10e: (i) State a chemical equation for the partial dissociation of water into ions, including...
- 13N.1.hl.TZ0.28: The table below shows data for the \({{K_{\text{a}}}}\) and \({{\text{p}}{K_{\text{b}}}}\)...
- 13N.2.hl.TZ0.7e.ii: Francisco and Shamiso found that the pH of the initial...
- 13M.1.hl.TZ1.26: The values of \({K_{\text{w}}}\), the ionic product constant of water, are: Which...
- 13M.1.hl.TZ1.27: For which equilibrium can an expression for a base dissociation constant, \({K_{\text{b}}}\),...
- 13M.1.hl.TZ2.28: The \({\text{p}}{K_{\text{b}}}\) value of ammonia is 4.75 at 298 K. What is the...
- 13M.1.hl.TZ2.29: The \({K_{\text{a}}}\) values of four weak acids W, X, Y and Z are listed below. W ...
- 13M.2.hl.TZ2.6a: Ammonia, NH3, can be used to clean ovens. The concentration of hydroxide ions, OH–(aq), in a...
- 13M.2.hl.TZ2.6d.iii: \({\text{150 c}}{{\text{m}}^{\text{3}}}\) of...
- 10N.2.hl.TZ0.6b: (i) Distinguish between the terms strong and weak acid and state the equations used to...
- 09N.2.hl.TZ0.8a.iii: Calculate the concentration of the weak acid before the addition of any NaOH(aq).
- 09N.2.hl.TZ0.8e.i: Determine the pH of the solution.
- 09N.2.hl.TZ0.8e.ii: Calculate the base dissociation constant, \({K_{\text{b}}}\), for ammonia.
- 10M.2.hl.TZ1.3d.i: State an equation for the reaction of ethanoic acid with water.
- 10M.2.hl.TZ1.3d.ii: Calculate the pH of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid...
- 09M.1.hl.TZ1.29: What is the approximate pH of a \({\text{0.01 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia...
- 09M.2.hl.TZ1.2a.i: State the equation for the reaction of propanoic acid with water.
- 09M.2.hl.TZ1.2a.ii: Calculate the hydrogen ion concentration (in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\))...
- 09M.1.hl.TZ2.29: At the same concentration, which acid would have the lowest...
- 09M.1.hl.TZ2.26: \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of a NaOH solution of pH 12 is mixed with...
- 09M.1.hl.TZ2.28: The indicator, HIn is used in a titration between an acid and base. Which statement about the...
- 09M.1.hl.TZ2.27: Ammonia acts as a weak base when it reacts with water. What is the \({K_{\text{b}}}\)...
- 09M.2.hl.TZ2.2a.iii: Determine the hydrogen ion concentration and the pH of a...
- 09M.2.hl.TZ2.2a.i: Calculate the \({K_{\text{a}}}\) value of benzoic acid,...
- 09M.2.hl.TZ2.2a.ii: Based on its \({K_{\text{a}}}\) value, state and explain whether benzoic acid is a strong or...
- 11M.1.hl.TZ1.28: The \({K_{\text{b}}}\) value for a base is...
- 11M.1.hl.TZ2.27: Based on information in the table below, which acid is the strongest?
- 11M.2.hl.TZ2.5a.iv: Calculate the pH of a...
- 12M.1.hl.TZ2.24: Four aqueous solutions are listed below. W. ...
- 12M.2.hl.TZ2.8d: A solution of ammonia has a concentration of...
- 11N.1.hl.TZ0.27: Consider the equation for the dissociation of...
- 11N.2.hl.TZ0.4b.ii: Determine the concentration of \({\text{O}}{{\text{H}}^ - }{\text{(aq)}}\), in...
- 11N.2.hl.TZ0.4b.i: The \({\text{p}}{K_{\text{a}}}\) value of HOCl(aq) is 7.52. Determine the \({K_{\text{b}}}\)...
- 11N.2.hl.TZ0.4b.iii: Calculate the pH of the bleach.

