| Date | November 2013 | Marks available | 1 | Reference code | 13N.3.hl.TZ0.26 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Identify | Question number | 26 | Adapted from | N/A |
Question
Limonene is a chiral molecule. The enantiomer found in oranges is shown below.
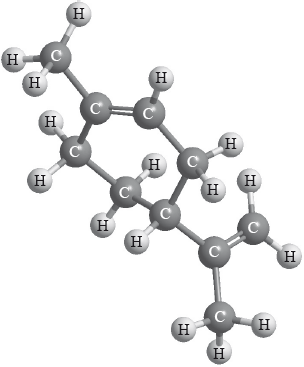
Identify the chiral carbon atom in the structure above with an asterisk, *.
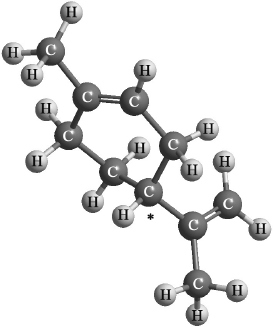
Markscheme
Examiners report
Option F was one of the less popular options. Identification of the two functional groups in the three antioxidants and why they contain tert- in the prefix to their name was generally done well. Strong candidates were able to provide the correct formula of BHT but many weaker candidates were not and this is a source of concern as it involves skills that should be basic in chemistry. Explanation of how natural and synthetic antioxidants act chemically in the process of auto-oxidation was generally done well. However, the mode of action of \({\text{S}}{{\text{O}}_{\text{2}}}\) as an antioxidant was not as successful as expected. The question on beta-carotene was done well.
Identification of the fatty acid with the highest melting point and the reason why was correctly answered by many but then many also failed to make use of intermolecular forces and therefore did not fully score. Another disappointing result was the equation for the complete hydrogenation of linolenic acid and once again related to difficulties in writing and balancing chemical equations. Many candidates were able to score the second mark by providing two correct conditions. The meaning of the term trans and the associated structure was answered very well.
Many correct answers were given for the meaning of a dispersed system but also many did not make use of subject specific vocabulary or repeated the word dispersed in the answer. The idea of an emulsion and foam was well understood as was the part on the structural features of an emulsifier.
Many candidates identified the chiral carbon atom correctly in the structure given and two different ways in which enantiomers might affect the properties of foods. However, the reason for the difference in optical activity when carvone is synthesized using limonene from natural or chemical source was poorly answered with many not scoring at all.

