| Date | May 2014 | Marks available | 6 | Reference code | 14M.3.sl.TZ1.10 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Deduce, Describe, and Suggest | Question number | 10 | Adapted from | N/A |
Question
Liquid crystals are widely used in devices such as calculators, laptop computers and advanced optical materials.
(i) Describe the meaning of the term liquid crystals and state which of the representations below (A, B or C) best describes molecules present in the liquid-crystalline phase.
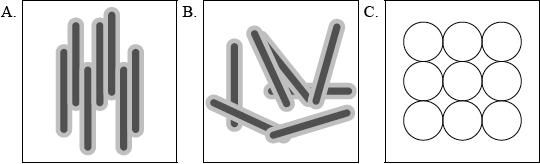
(ii) Deduce, with reasoning, which of the following substance(s) is/are most likely to show liquid-crystalline behaviour.
Substance I:
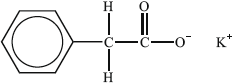
Liquid-crystalline behaviour (yes/no):
Reasoning:
Substance II:
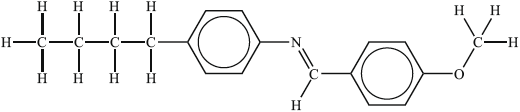
Liquid-crystalline behaviour (yes/no):
Reasoning:
Substance III:
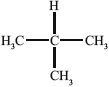
Liquid-crystalline behaviour (yes/no):
Reasoning:
(iii) Suggest why octane does not show liquid-crystalline behaviour.
(i) State one difference between thermotropic and lyotropic liquid crystals.
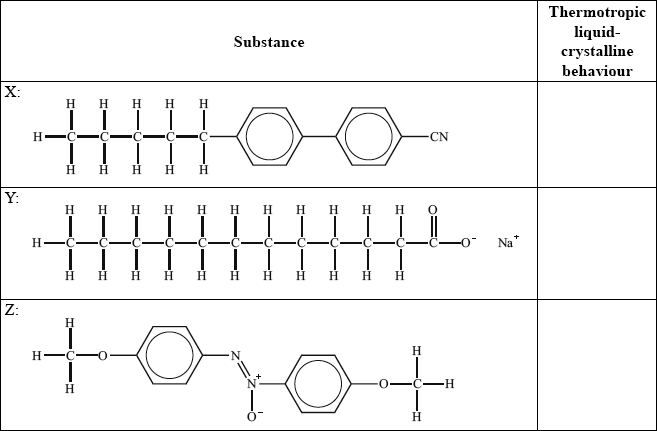
(ii) Identify, by stating yes or no, the substance(s) which show(s) thermotropic liquid crystalline behaviour.
Markscheme
(i) (LCs are) fluids that exhibit molecular orientation/orderly molecular arrangement and A;
Accept LCs show properties of liquids and crystals simultaneously and A.
(ii) I: no, since ionic (so high mp) / lacks long axis;
Allow no since it is not a molecule/not rod-shaped.
II: yes, since has long axis present (so limits ability of molecules to pack lowering mp);
Allow yes since rod-shaped.
has polar functional group / is polar (increasing intermolecular interactions) / (planar/flat) benzene ring present (assists stacking);
III: no, since lacks long axis;
Allow no since non-polar.
Allow no since not rod-shaped.
Award [1 max] for stating II only will show LC behaviour OR I: No, II: Yes and III: No.
Award [2 max] if one correct reason is given for each substance but LC behaviour is either incorrect or not given.
Award [3 max] if correct reasons are given for all three substances, but LC behaviour is either incorrect or not given.
(iii) (free) rotation about carbon-carbon single bonds (hence greater flexibility) so octane molecules not rod-shaped / OWTTE;
Do not allow mark for non-polar (molecule) only.
(i) Thermotropic: pure substances and lyotropic: solutions / thermotropic: show LC behaviour over limited temperature range (between solid and liquid states) and lyotropic: shows LC behaviour at certain concentrations;
(ii) X: yes and Y: no and Z: yes;
Award mark if no is stated only for Y or yes is only stated for X and Z.
Examiners report
This proved to be the most challenging question in option C. Candidates were able to identify A as the molecules present in the liquid-crystalline phase but were unable to describe the state for part (a)(i). Majority of the candidates were not able to score more than two marks for part (ii). Most were unable to identify substance I as ionic and substance II was often identified as ‘yes’ but without sound support. For (a)(iii), again students struggled with explicit sound reasoning. Response for (a)(iii) was poor with most stating non-polar as the reason for inability of octane to show liquid-crystalline behavior. Very few candidates were able to identify and explain if molecules will behave as a liquid crystal. Majority of the candidates were unable to score the point. Candidates were able to state the difference between the two liquid crystals in (b)(i) correctly but were not able to apply the information correctly in (b)(ii) to identify which substances shoe thermotropic liquid-crystalline behaviour.
This proved to be the most challenging question in option C. Candidates were able to identify A as the molecules present in the liquid-crystalline phase but were unable to describe the state for part (a)(i). Majority of the candidates were not able to score more than two marks for part (ii). Most were unable to identify substance I as ionic and substance II was often identified as ‘yes’ but without sound support. For (a)(iii), again students struggled with explicit sound reasoning. Response for (a)(iii) was poor with most stating non-polar as the reason for inability of octane to show liquid-crystalline behavior. Very few candidates were able to identify and explain if molecules will behave as a liquid crystal. Majority of the candidates were unable to score the point. Candidates were able to state the difference between the two liquid crystals in (b)(i) correctly but were not able to apply the information correctly in (b)(ii) to identify which substances shoe thermotropic liquid-crystalline behaviour.

