| Date | May 2015 | Marks available | 1 | Reference code | 15M.3.sl.TZ1.8 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | 8 | Adapted from | N/A |
Question
Thermotropic liquid crystals are widely used in display devices and sensors.
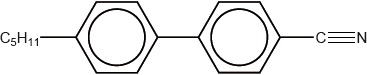
The structure of a material used in electrical display devices is shown below.
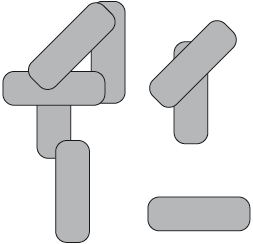
The diagram below shows eight molecules in the liquid state. Suggest, with a diagram, a possible arrangement that these rod-shaped molecules could adopt in the nematic liquid-crystal phase.
Suggest, with reference to the structure, why the molecule is able to change orientation in an electric field.
Suggest how the C5H11 chain contributes to the liquid-crystal properties of the compound.
Explain why a liquid-crystal device may be unreliable at low temperatures.
Markscheme
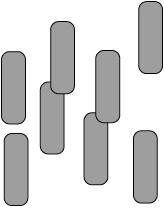
diagram should have molecules with a parallel alignment in any direction (not just upwards);
diagram should have molecules in an irregular arrangement in space;
Ignore relative separation between molecules.
Award [1 max] if number of molecules < 7.
Award [1 max] if stated “molecules align parallel to each other but with an irregular arrangement in space / OWTTE” but with no diagram drawn.
Allow the representation of molecules by lines.
polar/dipole moment due to the presence of C\( \equiv \)N (bond) / difference in electronegativity between C and N;
prevents close packing of molecules / OWTTE;
makes the molecule (longer and) more rod-like;
molecules become more ordered / molecules unable to change orientation (as they approach fixed arrangement of solid state) / molecules move slower / viscosity (of medium) increases (so LCD response time increases);
Examiners report
Well answered by many, although some candidates left the question blank. Candidates were more likely to align molecules in the same direction, but only some of them kept the arrangement of the molecules random and hence received the second mark.
The majority recognized that the alignment is due to the polarity, but only a few candidates attributed that to the CN bond.
Quite well answered about the role of the pentyl group in the liquid-crystal properties of the compound.
The better students were able to explain why a liquid-crystal device is unreliable at low temperature.

