| Date | May 2012 | Marks available | 2 | Reference code | 12M.3.hl.TZ1.C4 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | State | Question number | C4 | Adapted from | N/A |
Question
Kevlar behaves as a lyotropic liquid crystal when dissolved in suitable solvents. Its structure is shown below.
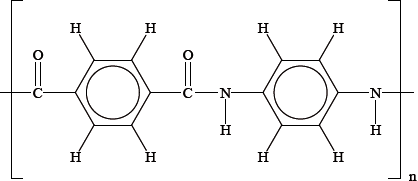
State the properties that a molecule, such as Kevlar, must have in order to enable it to behave as a liquid crystal.
Discuss the additional properties that a substance must have to make it suitable for commercial liquid-crystal displays.
Explain what is meant by the term lyotropic.
Markscheme
long rigid/rod-shaped molecules;
polar molecules / align with same orientation;
chemically stable;
liquid crystal phase over a suitable range of temperatures;
rapid switching speed;
(solution that only displays a liquid crystal state) over a range of/at certain concentrations;
Examiners report
A few students could identify some properties that liquid crystals must have.
Very few students were aware of the additional properties required for commercial application.
The meaning of lyotropic was reasonably well known.

