| Date | May 2015 | Marks available | 2 | Reference code | 15M.3.hl.TZ2.15 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain | Question number | 15 | Adapted from | N/A |
Question
Modern liquid crystals have a structure similar to this biphenyl nitrile.
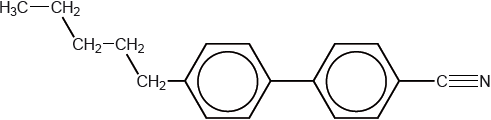
Explain how the structure of biphenyl nitriles makes them suitable for use in liquid-crystal devices.
Markscheme
biphenyl/planar ring structure makes molecule rigid/rod-shaped;
nitrile/cyano group makes molecule polar / allows orientation in an electric field / ensures alignment in common direction;
long hydrocarbon chain keeps molecules apart/prevents close packing/lowers melting point;
Award [1 max] for “chemically stable and rapid switching speed”.
Examiners report
In their answers students often failed to link specific structural elements of biphenyl nitriles with the desirable properties for an LCD material. The functioning of an LCD was however better known, with some quite detailed responses for which candidates often gained good credit. The mechanism for the generation of a potential difference by photovoltaic cells however seemed less well understood.

