| Date | May 2018 | Marks available | 3 | Reference code | 18M.2.hl.TZ1.4 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Describe | Question number | 4 | Adapted from | N/A |
Question
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The results of a series of experiments in which the concentration of HCl was varied are shown below.
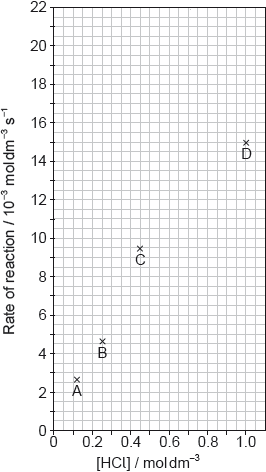
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Draw the best fit line for the reaction excluding point D.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Deduce the rate expression for the reaction.
Calculate the rate constant of the reaction, stating its units.
Predict from your line of best fit the rate of reaction when the concentration of HCl is 1.00 mol dm−3.
Describe how the activation energy of this reaction could be determined.
Markscheme
Any two of:
loss of mass «of reaction mixture/CO2»
«increase in» volume of gas produced
change of conductivity
change of pH
change in temperature
Do not accept “disappearance of calcium carbonate”.
Do not accept “gas bubbles”.
Do not accept “colour change” or “indicator”.
[2 marks]
reaction is fast at high concentration AND may be difficult to measure accurately
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
OR
insufficient change in conductivity/pH at high concentrations
OR
calcium carbonate has been used up/is limiting reagent/ there is not enough calcium carbonate «to react with the high concentration of HCl»
OR
HCl is in excess
OR
so many bubbles of CO2 produced that inhibit contact of HCl(aq) with CaCO3(s)
[1 mark]
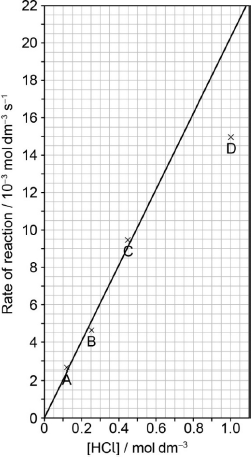
straight line going through the origin AND as close to A, B, C as is reasonably possible
[1 mark]
«directly» proportional
Accept “first order” or “linear”.
Do not accept “rate increases as concentration increases” or “positive correlation”.
[1 mark]
rate = k [H+]
Accept “rate = k [HCl]”.
[1 mark]
0.02
s–1
[2 marks]
20.5 \( \times \) 10–3 «mol dm–3 s–1»
Accept any answer in the range 19.5–21.5.
[1 mark]
ALTERNATIVE 1:
carry out reaction at several temperatures
plot \(\frac{1}{{\text{T}}}\) against log rate constant
Ea = – gradient \( \times \) R
ALTERNATIVE 2:
carry out reaction at two temperatures
determine two rate constants
OR
determine the temperature coefficient of the rate
use the formula \(\ln \frac{{{k_1}}}{{{k_2}}} = \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right)\)
Accept “gradient = \(\frac{{ - {E_{\text{a}}}}}{R}\)” for M3.
Award both M2 and M3 for the formula \({\text{ln}}\frac{{rat{e_1}}}{{rat{e_2}}} = \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_2}}} - \frac{1}{{{T_1}}}} \right)\).
Accept any variation of the formula, such as \(\frac{{rat{e_1}}}{{rat{e_2}}} = {e^{ - \frac{{{E_{\text{a}}}}}{R}\left( {\frac{1}{{{T_1}}} - \frac{1}{{{T_2}}}} \right)}}\).
[3 marks]

