| Date | November 2011 | Marks available | 4 | Reference code | 11N.2.hl.TZ0.8 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Calculate | Question number | 8 | Adapted from | N/A |
Question
\({\text{B}}{{\text{F}}_{\text{3}}}{\text{(g)}}\) reacts with \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) to form \({{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) according to the equation below.
\[{\text{B}}{{\text{F}}_3}{\text{(g)}} + {\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}} \to {{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\]
The following is a proposed mechanism for the reaction of NO(g) with \({{\text{H}}_{\text{2}}}{\text{(g)}}\).
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{\text{2NO(g)}} \to {{\text{N}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}} \\ {{\text{Step 2:}}}&{{{\text{N}}_2}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{N}}_2}{\text{O(g)}} + {{\text{H}}_2}{\text{O(g)}}} \end{array}\]
Identify the type of bond present between \({\text{B}}{{\text{F}}_{\text{3}}}\) and \({\text{N}}{{\text{H}}_{\text{3}}}\) in \({{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) and state another example of a compound with this type of bonding.
The table below shows initial rates of reaction for different concentrations of each reactant for this reaction at temperature, \(T\).
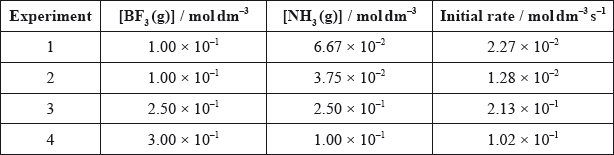
Deduce the rate expression, the overall order of the reaction and determine the value of \(k\), the rate constant, with its units, using the data from Experiment 4.
Identify the intermediate in the reaction.
The observed rate expression is \({\text{rate}} = k{{\text{[NO]}}^2}{\text{[}}{{\text{H}}_2}{\text{]}}\). Assuming that the proposed mechanism is correct, comment on the relative speeds of the two steps.
The following two-step mechanism has been suggested for the reaction of \({\text{N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) with CO (g), where \({k_2} \gg {k_1}\).
\[\begin{array}{*{20}{l}} {{\text{Step 1}}}&{{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{N}}{{\text{O}}_2}{\text{(g)}}\xrightarrow{{{k_1}}}{\text{NO(g)}} + {\text{N}}{{\text{O}}_3}{\text{(g)}}} \\ {{\text{Step 2:}}}&{{\text{N}}{{\text{O}}_3}{\text{(g)}} + {\text{CO(g)}}\xrightarrow{{{k_2}}}{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}} \\ {{\text{Overall:}}}&{{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}}\xrightarrow{{}}{\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}} \end{array}\]
The experimental rate expression is \({\text{rate}} = k{{\text{[N}}{{\text{O}}_2}{\text{]}}^2}\). Explain why this mechanism produces a rate expression consistent with the experimentally observed one.
HI(g) decomposes into \({{\text{H}}_2}{\text{(g)}}\) and \({{\text{I}}_{\text{2}}}{\text{(g)}}\) according to the reaction below.
\[{\text{2HI(g)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}}\]
The reaction was carried out at different temperatures and a value of the rate constant, \(k\), was obtained for each temperature. A graph of \(\ln k\) against \(\frac{1}{T}\) is shown below.
\(\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\)
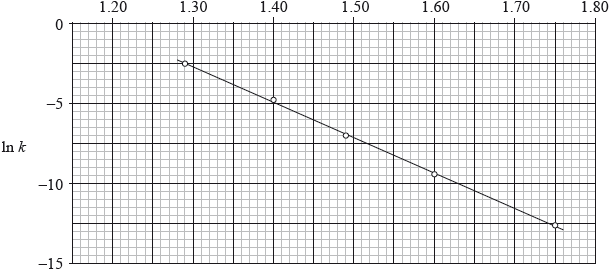
Calculate the activation energy, \({E_{\text{a}}}\), for the reaction using these data and Table 1 of the Data Booklet showing your working.
Markscheme
dative (covalent)/coordinate;
carbon monoxide/CO / hydronium (ion)/ \({{\text{H}}_3}{{\text{O}}^ + }\) / ammonium (ion)/\({\text{NH}}_4^ + \) / aluminium chloride/\({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\) / any relevant transition metal complex (e.g. \({{\text{[Ni(N}}{{\text{H}}_3}{{\text{)}}_6}{\text{]}}^{2 + }}\));
Accept AlCl3.
\({\text{rate}} = k{\text{[B}}{{\text{F}}_{\text{3}}}{\text{][N}}{{\text{H}}_{\text{3}}}{\text{]}}\);
second (order)/2°;
\(k = 3.40{\text{ d}}{{\text{m}}^{\text{3}}}{\text{mo}}{{\text{l}}^{ - 1}}{{\text{s}}^{ - 1}}\);
Allow units of L mol–1s–1 or M–1s–1.
Units required for mark.
\({{\text{N}}_2}{{\text{O}}_2}\);
(\({\text{[}}{{\text{H}}_2}{\text{]}}\) appears in rate expression so) step 2 rate-determining/rds/slow step;
Allow “since step 1 involves 2NO and step 2 involves H2 and as all 3 molecules are involved in rate expression, then two steps must have approximately same rate” / OWTTE.
(\({k_2} \gg {k_1}\) so) step 1 rate-determining/rds/slow step;
two molecules of \({\text{N}}{{\text{O}}_{\text{2}}}\) involved in step 1 consistent with rate expression / rate of overall reaction must equal rate of step 1 which is \({\text{rate}} = {k_1}{{\text{[N}}{{\text{O}}_{\text{2}}}{\text{]}}^{\text{2}}}\) / OWTTE;
\({E_{\text{a}}} = - R \times m\);
measurement of gradient from two points on line;
Accept a gradient in range –2.14 \( \times \) 104 K to –2.27 \( \times \) 104 (K).
correct answer for \({E_{\text{a}}}\);
correct units \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{/J}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) corresponding to answer;
Allow kJ or J.
A typical answer for Ea = 1.85 \( \times \) 102 kJ\(\,\)mol–1.
Allow answers for Ea in range 1.75 \( \times \) 102 kJ\(\,\)mol–1 to 1.91 \( \times \) 102 kJ\(\,\)mol–1.
Award [4] for correct final answer with some working shown.
Award [2 max] for correct final answer without any working shown.
Examiners report
(i) was usually well done.
some did not answer the question which asked for the overall order of the reaction. Some candidates also got their units incorrect. A few G2 comments mentioned the fact that the data was quite complicated as there was no experiment with \({\text{[N}}{{\text{H}}_{\text{3}}}{\text{]}}\) constant. It is true that the maths here may appear more challenging than normal, but candidates should be able to handle this type of data and in fact a significant number of the better candidates did score full marks on this question.
This was well answered.
This was well answered.
Although most candidates stated that the rds was step 1, many struggled with the explanation.
Although this question has been asked on a number of recent papers, candidates really struggled with this graphical based format. All sorts of mistakes were made, including gradients, units etc. Some did not even know how to approach the question.

