| Date | November 2014 | Marks available | 1 | Reference code | 14N.1.hl.TZ0.21 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Question number | 21 | Adapted from | N/A |
Question
What happens to the rate constant, k, and the activation energy, \({E_{\text{a}}}\), as the temperature of a chemical reaction is increased?
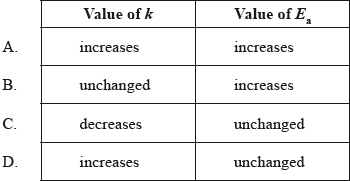
Markscheme
D
Examiners report
[N/A]

