

Nucleus
The Thomson model of the atom had the following key ideas:
- A solid lump of homogeneous positive charge (the "pudding")
- Small negative regions located randomly throughout (the "plums")
- Neutral overall

This model explained the existence of discrete particles of negative charge (electrons) but was proved wrong by Rutherford.
Electrons and quantum
So far we have considered light to behave as a wave. However, light also behaves as discrete quantised particles (photons) in certain experiments. Energy and wavelengths of light are linked mathematically so that specific wavelengths correspond to specific energies:
\(E=hf\) and \(E={hc\over \lambda}\)
- E = energy of a photon (J)
- h = Planck's constant (\(6.63 \times 10^{-34}\) m2 kg s-1)
- f = frequency of light (Hz)
- c = speed of light (\(3\times 10^8\)ms-1)
- λ = wavelength of light (m)
How much of Atomic models have you understood?



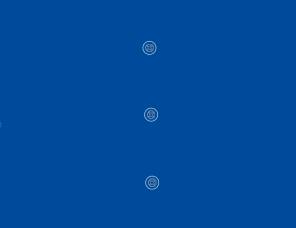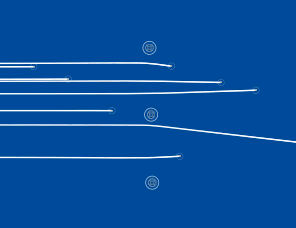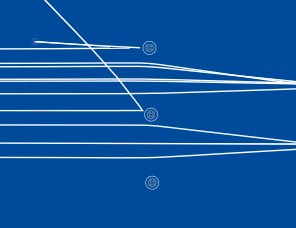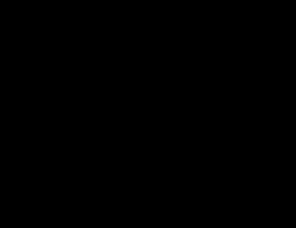

 The line spectra of low pressure gases can be explained if the electrons can only have certain discrete energy levels. These can be represented on an energy level diagram. This is the energy level diagram for hydrogen.
The line spectra of low pressure gases can be explained if the electrons can only have certain discrete energy levels. These can be represented on an energy level diagram. This is the energy level diagram for hydrogen. We have a lot of evidence that electrons exist in discrete energy levels.
We have a lot of evidence that electrons exist in discrete energy levels. Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn