Respiration & Carbon Dioxide
- All living cells carry out some form of cellular respiration, from bacteria, to plants, to animals
- Many organisms carry out aerobic respiration
- Aerobic respiration requires the uptake of oxygen and produces carbon dioxide as a waste product
Aerobic respiration produces carbon dioxide as a waste product
- Because carbon dioxide is being constantly produced inside cells, a concentration gradient between the inside and the outside of cells is maintained
- Carbon dioxide leaves cells by diffusion
- This carbon dioxide is eventually released into the environment; either air or water
- Single-celled organisms release carbon dioxide by diffusion at the cell surface
- Terrestrial plants release carbon dioxide by diffusion from their stomata into the surrounding air
- Animals release carbon dioxide into the surrounding air or water by diffusion via their gas exchange surfaces e.g. mammalian lungs or fish gills
Fluctuations in Carbon Dioxide Levels
NOS: Making accurate, quantitative measurements; it is important to obtain reliable data on the concentration of carbon dioxide and methane in the atmosphere
- The amount of carbon in the atmosphere is constantly changing due to seasonal fluctuations in rates of photosynthesis and due to human activities
- Photosynthesis removes carbon dioxide from the atmosphere, meaning that atmospheric carbon dioxide levels decrease in whichever hemisphere is experiencing spring and summer
- The combustion of fossil fuels by humans releases carbon dioxide into the atmosphere
- Livestock such as cattle release methane into the atmosphere
- Both carbon dioxide and methane gases contribute to atmospheric carbon levels, and both of these gases have important impacts on the planet
- Carbon dioxide influences the pH of seawater and the process of photosynthesis, and both carbon dioxide and methane influence global temperatures
- Because of the significance of the effects of these gases, it is important to accurately monitor their concentrations in the atmosphere
- Quantitative atmospheric measurements can be taken
- Accurate, quantitative monitoring over a long period of time enables scientists to identify trends, and test hypotheses
- E.g. Scientists have hypotheses such as:
- Increased atmospheric carbon dioxide is due to human activities
- Increased carbon dioxide causes increasing global temperatures
- Data enables scientists to see that levels of atmospheric carbon have increased in line with human burning of fossil fuels, and that increasing atmospheric carbon levels correspond with increasing global temperatures
- E.g. Scientists have hypotheses such as:
- Quantitative data enables scientists to make predictions
- E.g.
- Studying the connection between atmospheric carbon levels and global temperatures enables scientists to predict the level of impact of the release of specific amounts of carbon into the atmosphere
- Understanding the impact of global photosynthesis rates on atmospheric carbon levels means that scientists can accurately predict the impact of projects such as large-scale tree planting and rewilding
- E.g.
- Statistical analysis can be carried out on quantitative data, enabling statistical significance to be established
- E.g, scientists can be sure that current carbon dioxide levels are significantly higher than they would have been without the combustion of fossil fuels
- Scientists from the World Meteorological Organisation and research stations (e.g. the Mauna Loa Observatory) have been taking quantitative measurements of the atmospheric carbon dioxide and methane concentrations for many years
- Scientists have records for carbon dioxide levels dating back to 1958, and for methane levels from 1984
- It is possible for scientists to find information about concentrations of atmospheric gases over a longer period of time by analysing gas bubbles from deep ice cores
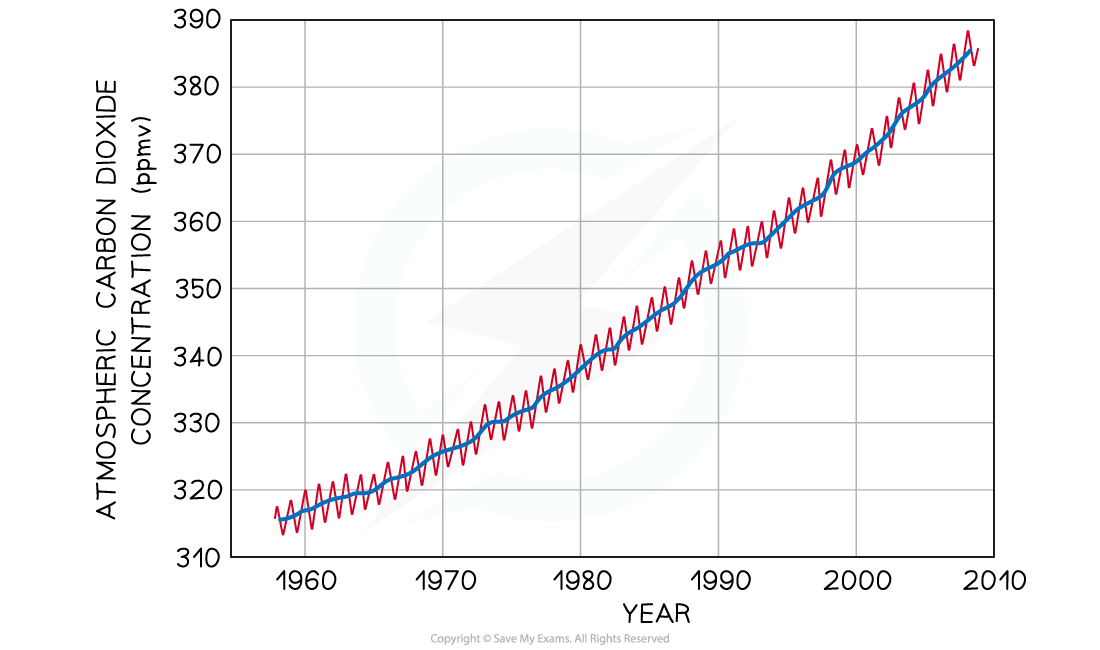
Changes in atmospheric carbon dioxide levels measured at the Mauna Loa Observatory (ppmv = parts per million by volume). The yearly fluctuations shown in red are due to seasonal changes in photosynthesis rates.
Combustion
- Carbon can be returned to the atmosphere by the burning of fossil fuels and organic material; a process known as combustion
- Complete combustion releases carbon dioxide and water as byproducts
- Fossil fuels form when animals and plants die in conditions where decomposing microorganisms are not present; the carbon in their bodies is converted, over millions of years and with significant pressure, into fossil fuels such as coal and oil
- The combustion of fossil fuels releases carbon that has been stored for millions of years
- Increased use of fossil fuels is contributing to an increase in the carbon dioxide content of the atmosphere
- Organic material, or biomass, burns when fires occur in e.g. forests or grasslands
- Such fires can have natural causes e.g. lightning hitting hot, dry ground, or can be set by humans e.g. when clearing land for the purpose of farming
- Biomass can also be burned as a fuel in e.g. wood fires or biomass boilers
