Properties of Water
NOS: Use theories to explain natural phenomena; the theory that hydrogen bonds form between water molecules explains the properties of water
- When scientists observe natural phenomena they try to come up with credible theories to explain their occurrence
- A theory is presumed to be correct if it explains the observation, is supported by experimental evidence and has not been falsified
- As hydrogen bonds are not visible, scientists can never say for certain that they exist, however there is strong experimental evidence to suggest that they do
- The presence and number of hydrogen bonds between polar water molecules helps to explain water's unique properties
The properties of water
Solvent
- As water is a polar molecule many ions (e.g. sodium chloride) and covalently bonded polar substances (e.g. glucose) will dissolve in it
- This allows chemical reactions to occur within the cytoplasm of cells (as the dissolved solutes are more chemically reactive when their individual molecules are free to move about)
- Metabolites can be transported efficiently (except non-polar molecules which are hydrophobic)
- Water molecules 'surround' individual solute particles to ensure each solute particle is isolated from others
- This explains why solutions are clear - we can't see individual molecules that are separated from their crystal structures
- This is also why concentrated solutions have a lower water potential or a higher osmolarity
- Because many water particles are 'occupied' in keeping a solute molecule in solution, fewer water molecules are free to diffuse across partially permeable membranes
Water has a high specific heat capacity
- Specific heat capacity is a measure of the energy required to raise the temperature of 1 kg of a substance by 1oC
- Water has a high specific heat capacity of 4200 J/kg/oC meaning a relatively large amount of energy is required to raise its temperature
- The high specific heat capacity is due to the many hydrogen bonds present in water
- It takes a lot of thermal energy to break these bonds and a lot of energy to build them, thus the temperature of water does not fluctuate greatly
- The advantage for living organisms is that it:
- Provides suitable, stable habitats
- Is able to maintain a constant temperature as water is able to absorb a lot of heat without wide temperature fluctuations
- This is vital in maintaining temperatures that are optimal for enzyme activity
- Water in blood plasma is also essential in transferring heat around the body, helping to maintain a fairly constant temperature, especially at body extremities eg. fingertips
- As blood passes through more metabolically active (‘warmer’) regions of the body, heat energy is absorbed but the temperature remains fairly constant
- Water in tissue fluid also plays an important regulatory role in maintaining a constant body temperature
Water has a high latent heat of vaporisation
- In order to change state (from liquid to gas) a large amount of thermal energy must be absorbed by water to break the hydrogen bonds and allow individual gas particles to escape (evaporate)
- This explains water's high boiling point (100°C)
- Water is present on Earth in all three physical states (solid, liquid and gas) thanks to this characteristic
- Ice, liquid water and water vapour all play a vital role in the biosphere
- This is an advantage for living organisms as only a little water is required to evaporate for the organism to dissipate a great amount of heat
- This provides a cooling effect for living organisms, for example, the transpiration from leaves or evaporation of water in sweat from the skin
Properties of Water & its Role in Living Organisms Table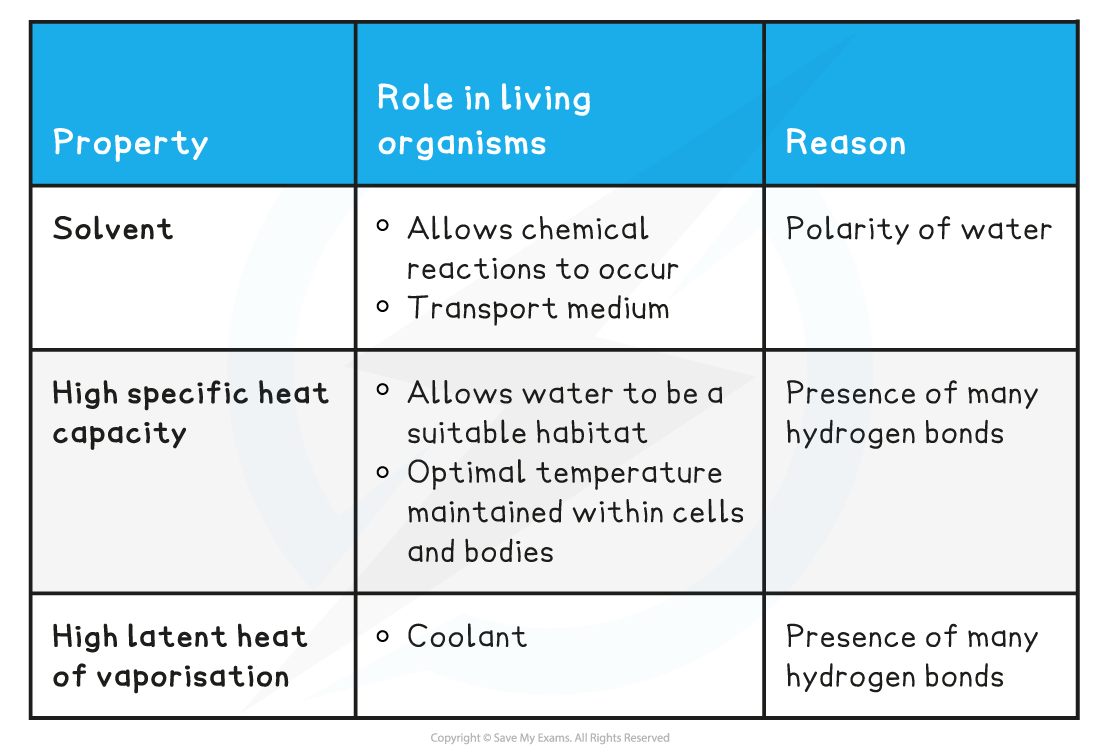
Cohesion and adhesion
- Hydrogen bonds between water molecules allows for strong cohesion between water molecules
- Allowing columns of water to move (called mass transport) through the xylem of plants and through blood vessels in animals
- Enabling surface tension where a body of water meets the air, these hydrogen bonds occur between the top layer of water molecules to create a sort of film on the body of water
- This layer is what allows insects such as pond skaters to move across the surface of water
- Water is also able to hydrogen bond to other molecules, such as cellulose, which is known as adhesion
- This also enables water to move up the xylem during transpiration
- Cohesion and adhesion both contribute to water forming a meniscus in glassware, where water molecules adhere to polar molecules in the glass
- Water adheres to the xylem walls (made of lignin) by capillary action
Exam Tip
COhesion = water particles sticking to each otherADhesion = water particles sticking to other materials
Hydrophilic & Hydrophobic
- Biological molecules can be hydrophilic or hydrophobic (and sometimes both)
- Hydrophilic = "water-loving"
- Hydrophobic = "water-hating"
- Polar molecules and molecules with positive or negative charges can form hydrogen bonds with water (and dissolve) so are generally hydrophilic
- Non-polar molecules with no positive or negative charge, cannot form hydrogen bonds with water so are generally hydrophobic
- These molecules tend to join together in groups due to hydrophobic interactions where hydrogen bonds form between water particles but not with the non-polar molecule
- Because most biological molecules are hydrophilic and can be dissolved, water is regarded as the universal solvent
- Some large molecules have different groups with different characteristics
- Phospholipids have hydrophilic (phosphate group) heads and hydrophobic (hydrocarbon chain) tails. This dual character is a key feature in the structure and function of cell membranes
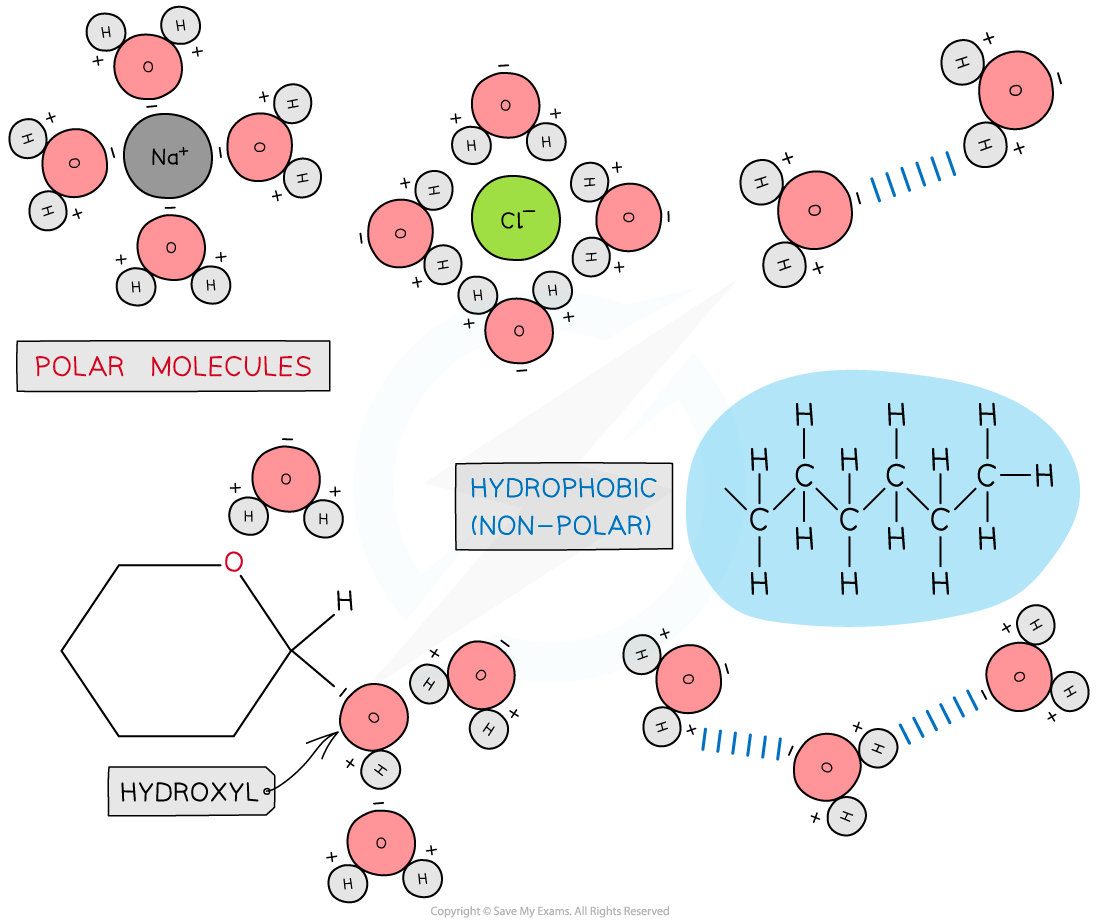
Due to its polarity water is considered a universal solvent
Focus on Water as a Coolant
- Water's high latent heat of vaporisation makes it an excellent coolant
- Animals have evolved sweating (perspiration) as a way of disposing of excess heat generated through physical activity
- The hypothalamus detects changes to blood temperature and when temperatures rise, it stimulates the secretion of sweat
- Small droplets of water are secreted from sweat glands onto the skin's surface
- Vasodilation of arterioles just beneath the skin carries more blood close to the surface
- Sweat (mainly water, also contains salts and other solutes) evaporates, carrying the excess heat away into the surrounding air and reducing the temperature of the organism
- Water's high latent heat of vaporisation allows only small volumes of water to be needed to carry away a lot of heat
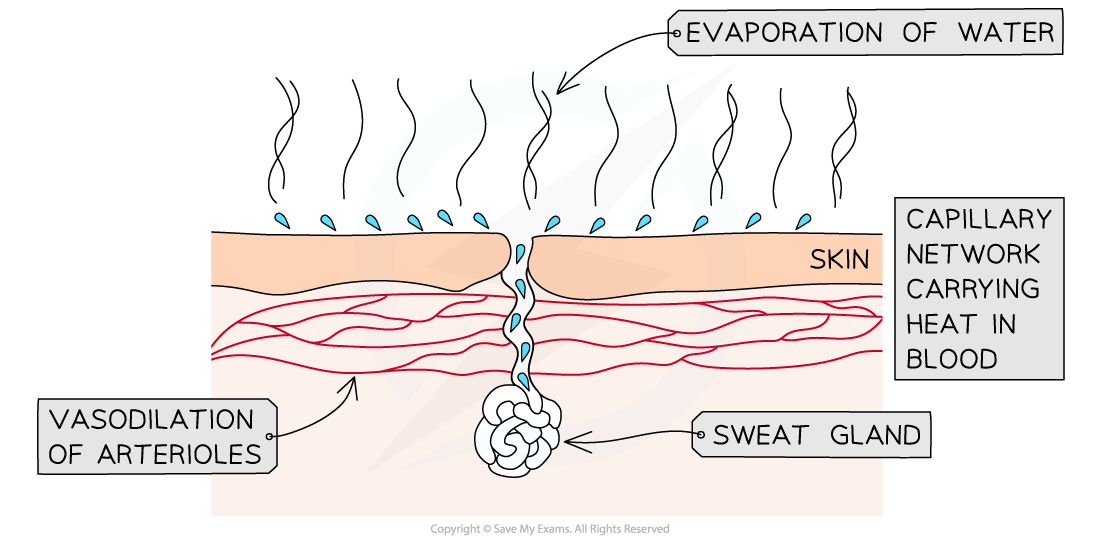
The excess heat carried in blood causes the evaporation of sweat from the skin surface
Water as a coolant in plants
- Plants transpire
- A large tree will stand in direct sunlight all day, so will absorb a huge amount of heat (as infra-red radiation from the Sun) on a hot day
- A tree cannot seek shade, because it requires light energy for photosynthesis
- A tree is also immobile and provide shade for other organisms
- A transpiration stream of water flows up the tree, from roots to xylem to leaves, throughout the day
- Water evaporates inside the spongy mesophyll layer of leaves, so water vapour can diffuse out via the stomata
- For example, a large oak tree can absorb around 500 litres of water per day from the soil, around 90% of which is evaporated in transpiration to dissipate heat
- The remainder is used to keep cells turgid and as a raw material for photosynthesis
Exam Tip
Sweat and transpiration have a lot of parallels in keeping animals and plants cool. This is why the French use the same word for both; the French word for "sweating" is "transpiration"!
Focus on Water as a Solvent
- Different solutes behave differently with water as a solvent
- Even though water is a universal solvent, different metabolites have different solubilities in water
- Different solutes have different hydrophobic and hydrophilic properties which affect their solubility in water
Highly soluble metabolites
- Some are highly soluble (eg. sodium chloride, urea), some are insoluble (eg. fats) and some have intermediate solubility (eg. oxygen and certain amino acids with a large R group)
- Highly soluble metabolites simply travel dissolved in the blood plasma
- eg. salts, glucose, amino acids
- Even the amino acids with hydrophobic R groups are soluble enough to be freely transported in water
- eg. salts, glucose, amino acids
- Different transport mechanisms have evolved to assist in the transportation of the less soluble metabolites
Less soluble metabolites
- A low solubility metabolite such as oxygen requires assistance through combining with haemoglobin, to allow more oxygen to be carried than directly in blood plasma
- Oxygen is less soluble at body temperature (37ºC) than at 20ºC
- Oxygen is sparingly soluble but soluble enough to allow enough to dissolve in oceans, rivers and lakes for aquatic animals to breathe
- Haemoglobin can bind oxygen to allow sufficient oxygen to be transported to all body cells
- Insoluble metabolites like fats require emulsification, and transport in lacteals, or by being converted to soluble phospholipids
- Cholesterol, which is insoluble, is converted to lipoproteins by combining with proteins
