Structure of Enzymes
- Enzymes are biological catalysts
- ‘Biological’ because they function in living systems
- ‘Catalysts’ because they speed up the rate of chemical reactions without being used up or changed themselves
- Enzymes have an active site to which specific substrates bind
- Enzymes are also globular proteins
- Critical to the enzyme's function is the active site where the substrate binds
- Enzymes are specific to the substrate
- The shapes of the enzyme and substrate and their chemical properties are complementary, to allow the substrate to fit into the active site, like two jigsaw pieces fitting together
- This is called enzyme-substrate specificity
- Due to this specificity, thousands of enzymes are needed throughout an organism, to carry out individual chemical reactions
Enzyme Activity
- Enzyme catalysis involves molecular motion and the collision of substrates with the active site
- For an enzyme-catalysed reaction to take place, substrates collide at random with the enzyme's active site
- This must happen at the correct orientation and speed in order for a reaction to occur
- Unsuccessful collisions can occur when the molecules are not correctly aligned with each other at the moment of collision
- The molecules 'bounce' off each other and no reaction takes place
- Some enzymes have two substrates that must each collide with a separate active site at the same time
- Substrates bind to enzymes, forming a temporary enzyme-substrate complex
- The active site of an enzyme has a specific shape and chemical properties to bind with a specific substrate
- The reaction occurs within the enzyme-substrate complex which leads to changes in the chemical structure of the substrate
- Products are formed, which detach and move away from the active site, which can be re-used
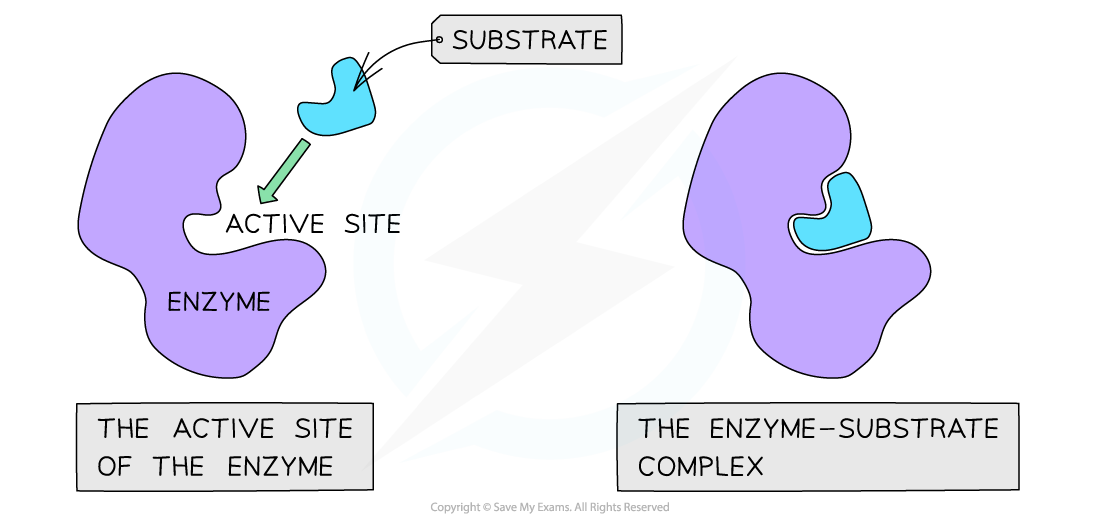
The active site of an enzyme has a specific shape to fit a specific substrate (when the substrate binds an enzyme-substrate complex is formed)
- The specificity of an enzyme is a result of the complementary nature between the shape of the active site on the enzyme and its substrate(s)
- The shape of the active site (and therefore the specificity of the enzyme) is determined by the complex 3-D shape of the protein that makes up the enzyme
- Proteins are formed from chains of amino acids held together by peptide bonds
- The order of amino acids in this chain determines the shape of an enzyme
- If the order is altered, the resulting three-dimensional shape changes
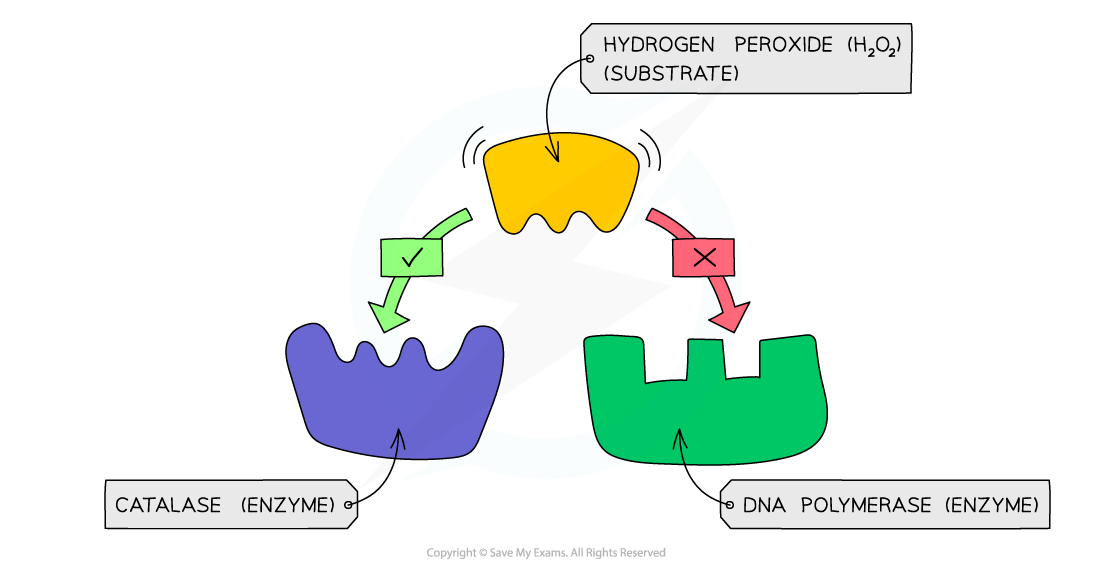
An example of enzyme specificity – the enzyme catalase can bind to its substrate hydrogen peroxide as they are complementary in shape, whereas DNA polymerase is not
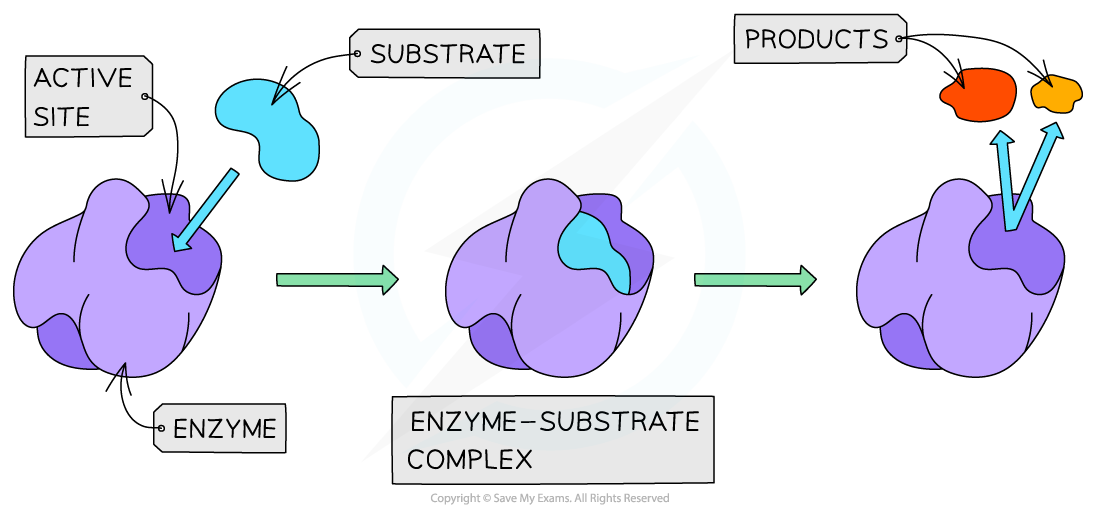
The temporary formation of an enzyme-substrate complex
- Enzyme reactions can either be catabolic or anabolic
- Catabolic reactions involve the breakdown of complex molecules into simpler products, which happens when a single substrate is drawn into the active site and broken apart into two or more distinct molecules
- Examples of catabolic reactions include cellular respiration and hydrolysis reactions
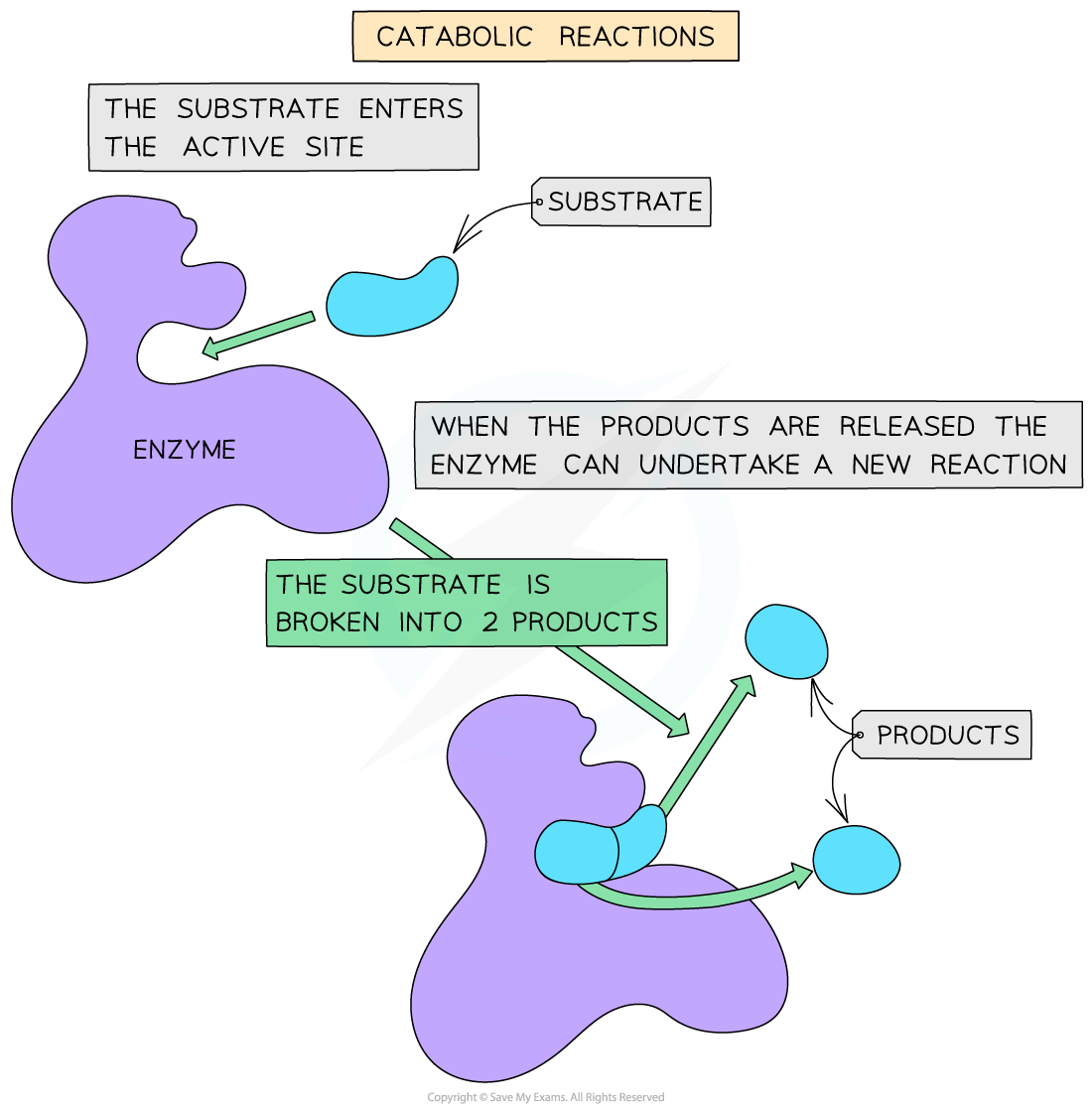
A catabolic reaction
- Anabolic reactions involve the building of more complex molecules from simpler ones when two or more substrates are held in the active site, forming bonds between them and releasing a single product
- Examples of anabolic reactions include protein synthesis and photosynthesis
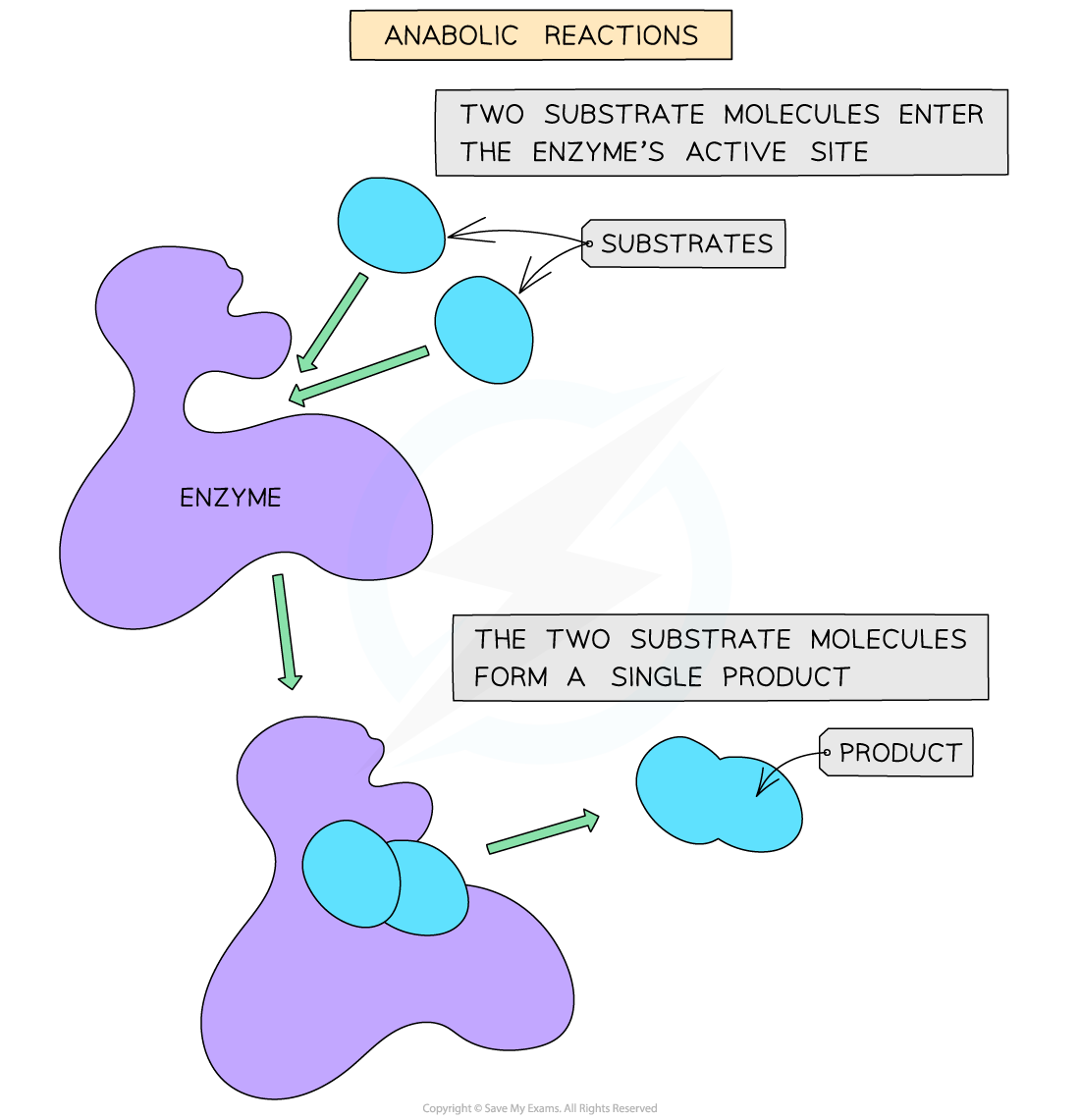
An anabolic reaction
Exam Tip
Don't forget that both enzymes and their substrates are highly specific to each other – this is known as enzyme-substrate specificity.
Factors Affecting Enzyme Activity
- Temperature, pH and substrate concentration affect the rate of activity of enzymes
- Enzymes have a specific optimum temperature – the temperature at which they catalyse a reaction at the maximum rate
- Lower temperatures either prevent reactions from proceeding or slow them down:
- Molecules move relatively slowly
- Lower frequency of successful collisions between a substrate molecule and the active site of enzyme
- Less frequent enzyme-substrate complex formation
- Substrate and enzyme collide with less energy, making it less likely for bonds to be formed or broken (stopping the reaction from occurring)
- Higher temperatures speed up reactions:
- Molecules move more quickly
- Higher frequency successful collisions between a substrate molecule and the active site of enzyme
- More frequent enzyme-substrate complex formation
- Substrate and enzyme collide with more energy, making it more likely for bonds to be formed or broken (allowing the reaction to occur)
- However, as temperatures continue to increase, the rate at which an enzyme catalyses a reaction drops sharply, as the enzyme begins to denature
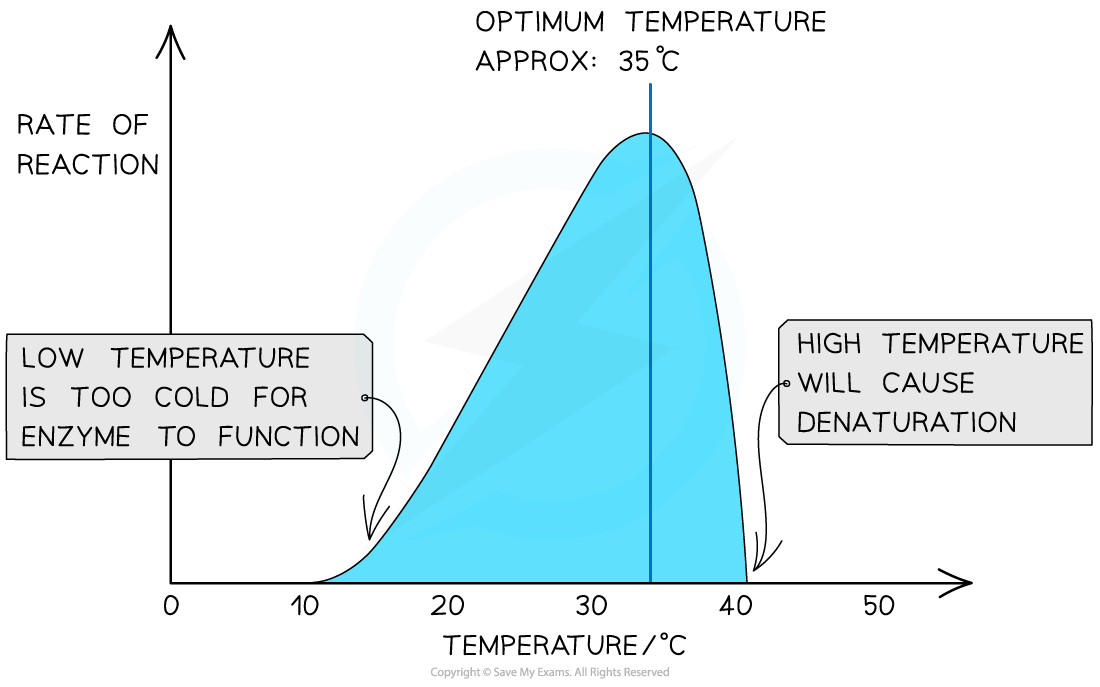
The effect of temperature on the rate of an enzyme-catalysed reaction
Changes in pH
- pH is a result of the hydrogen ion concentration in a solution
- A low pH is acid and has a high hydrogen ion concentration
- A high pH is alkaline and has a low hydrogen ion concentration
- A 10× increase in hydrogen ion concentration lowers the pH by 1 unit
- pH is therefore measured on a logarithmic scale of hydrogen ion concentration, not a linear scale
- Water has a pH of 7, regarded as neutral
- Extremes of pH can also alter hydrogen bonding within an enzyme's structure and cause irreversible denaturation
- Each enzyme has an optimum pH
- Not all enzymes have an optimum pH near to neutral. For example
- The stomach enzyme pepsin is adapted to work best at pH 2
- Certain bacterial enzymes work at pH 9-10, in line with the pH of the bacteria's main habitat
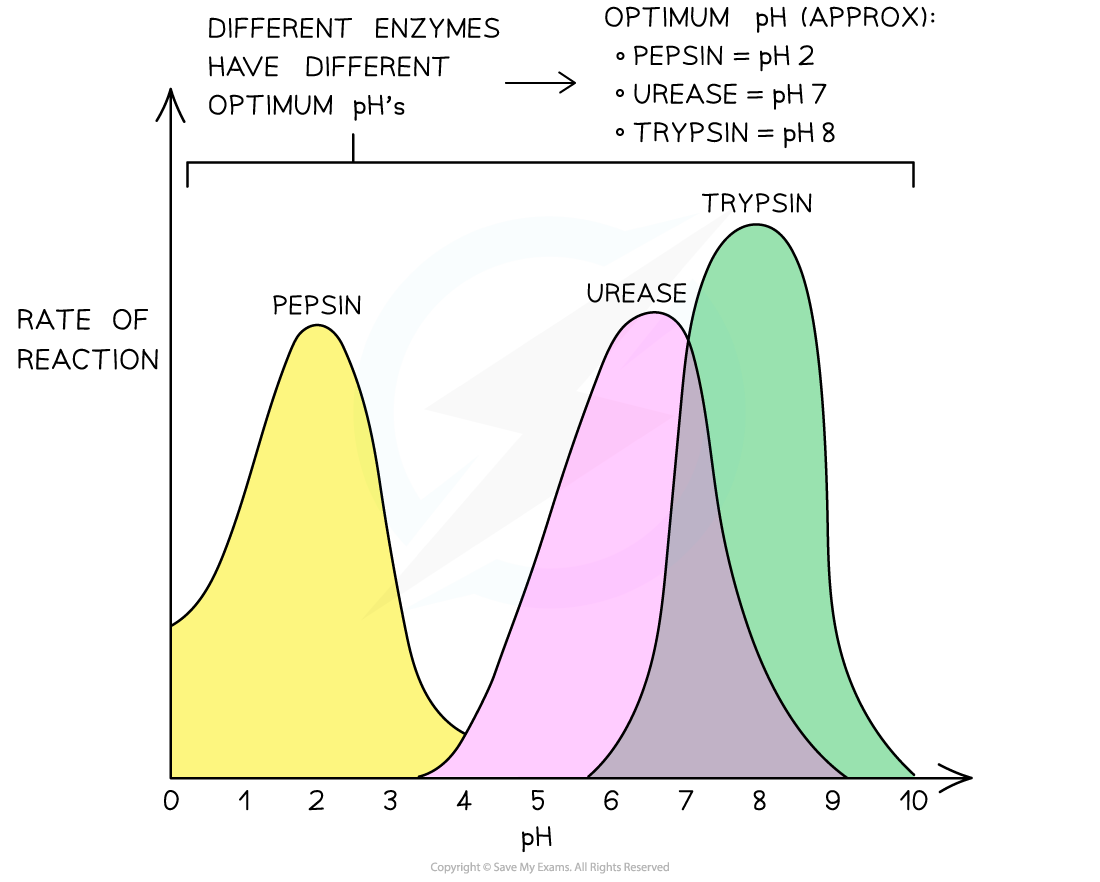
The effect of pH on three enzymes' rates of reaction
Changes in substrate concentration
- The more substrate molecules are present in a solution, this increases the frequency of collisions with the enzyme's active site
- Active sites are occupied or 'blocked' by substrates whilst the reaction is taking place
- The more active sites are occupied, the fewer are available to catalyse other substrate molecules
- As substrate concentration rises, the slower the rise in the rate of the enzyme-catalysed reaction
- The active sites have become saturated
- At the point of active site saturation, increasing the substrate concentration will cause no further increase in the rate of reaction
- At the point of active site saturation, a method of increasing the rate of reaction would be to make more active sites available by increasing the enzyme concentration
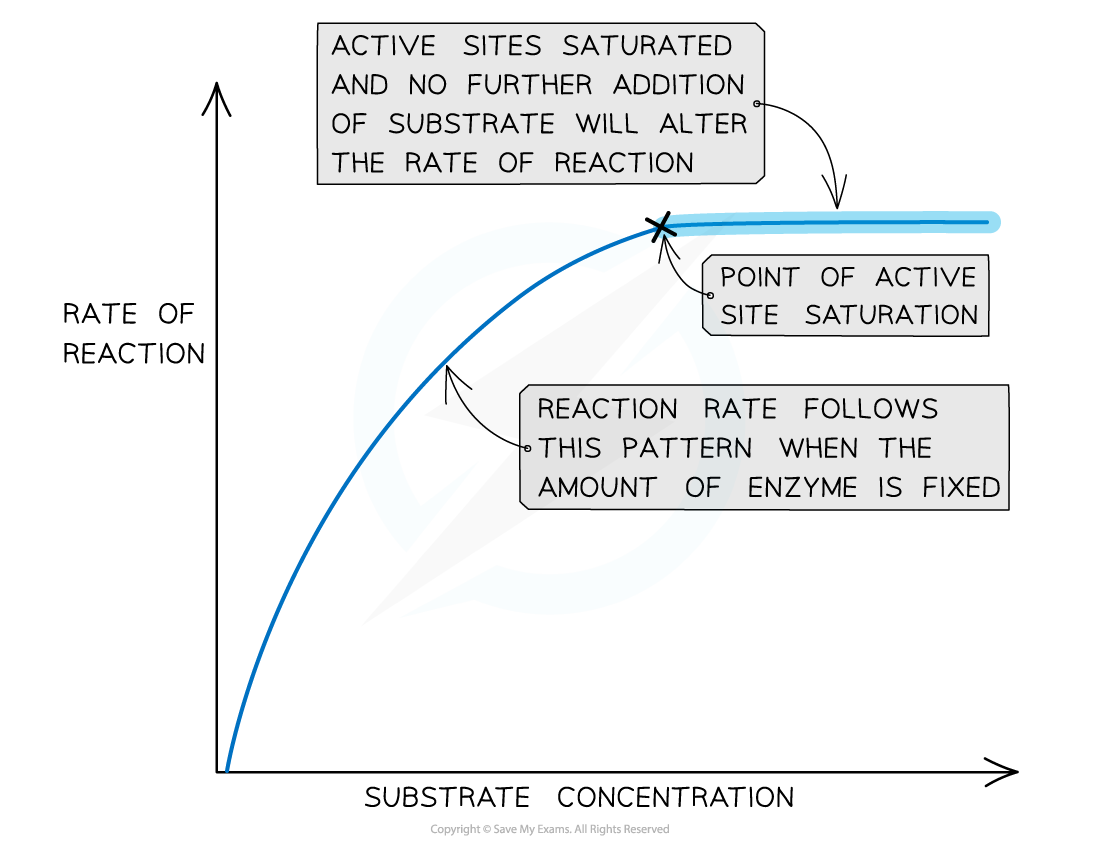
The effect of substrate concentration on enzyme activity
Exam Tip
When answering questions about reaction rates for enzyme-catalysed reactions, make sure to explain how the temperature affects the speed at which the molecules (enzymes and substrates) are moving and how this, in turn, affects the number of successful collisions.You should memorise the sketch graphs of temperature, pH and substrate concentration and be able to sketch new curves for changed conditions.
Denaturation: Enzymes
- Enzymes can be denatured
- High temperatures and extremes of pH cause denaturation
- Bonds (eg. hydrogen bonds) holding the enzyme molecule in its precise 3D shape start to break
- This causes the 3-dimensional shape of the protein (ie. the enzyme) to change
- This permanently damages the active site, preventing the substrate from binding
- Denaturation has occurred if the substrate can no longer bind
- The reaction that was previously catalysed now no longer takes place
- Denaturation often causes the enzyme to become insoluble and form a precipitate
- Very few human enzymes can function at temperatures above 50°C
- This is because humans maintain a body temperature of about 37°C, therefore even temperatures exceeding 40°C will cause the denaturation of enzymes
- High temperatures cause increased vibrations in the bonds and the hydrogen bonds between amino acids start to break, changing the conformation of the enzyme
Exam Tip
Don't forget that enzymes are always proteins and so anything that could denature a protein, rendering it non-operational (extremes of heat, temperature, pH etc.) would also denature an enzyme.Avoid using the term 'destroyed' when describing the disruption to enzyme structure; the more accurate term is 'denatured'.
