Practical 3: Enzyme Experiments
Design of experiments to test the effect of temperature, pH and substrate concentration on the activity of enzymes
- Three different independent variables can be tested
- Temperature
- pH
- Substrate concentration
- You should plan how the dependent variable is going to be measured
- With appropriate units
- Also, what intervals of the independent variable are going to be chosen
- These factors dictate the choice of apparatus and other equipment required for the experiment
- The control variables need to be identified and monitored eg. temperature when measuring the effect of pH
Investigating the effects of temperature or pH on catalase activity
- The progress of enzyme-catalysed reactions can be investigated by:
- Measuring the rate of formation of a product
- Measuring the rate of disappearance of a substrate
- In this investigation, the rate of product formation is used to measure the rate of an enzyme-controlled reaction:
- Hydrogen peroxide is a common but toxic by-product of metabolism
- This means it must be broken down quickly
- Catalase is an enzyme found in the cells of most organisms that breaks down hydrogen peroxide into water and oxygen
- Hydrogen peroxide and catalase are combined and the volume of oxygen generated is measured in a set time
- The rate of reaction can then be calculated
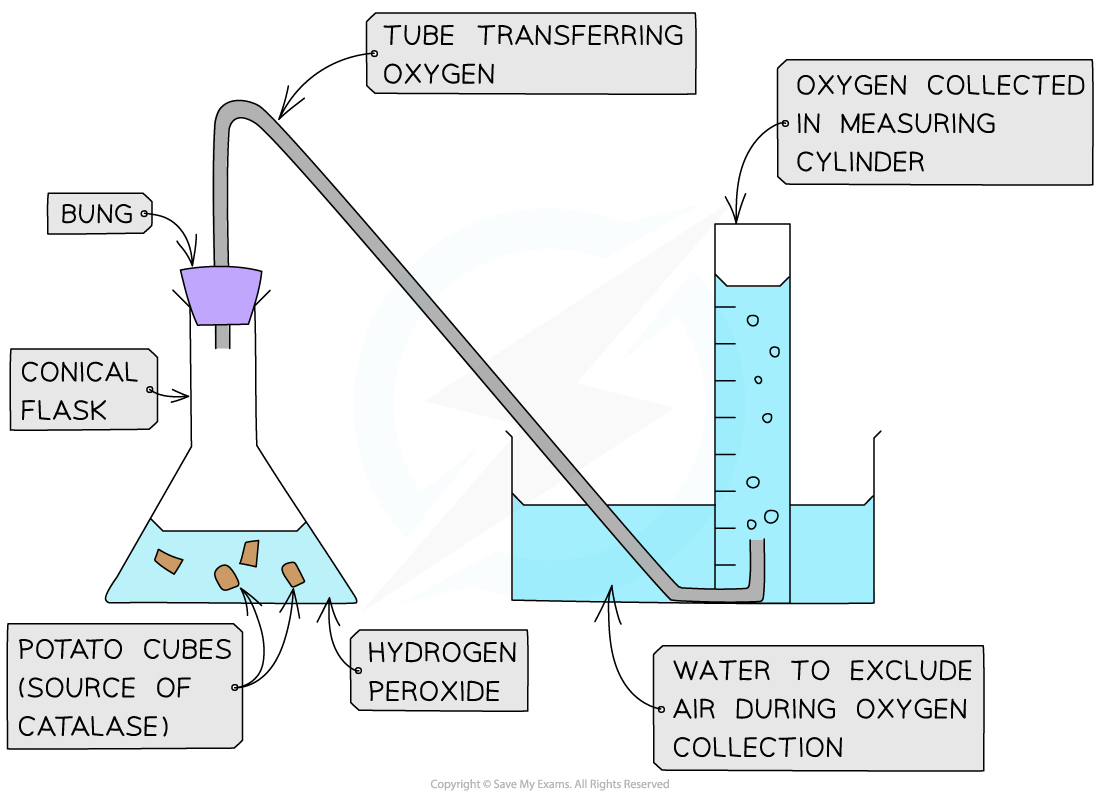
Experimental set-up for investigating the rate of formation of a product using catalase
- If measuring the effect of temperature on enzyme activity, the conical flask containing potato pieces can be held in a water bath at the required temperature
- The water level in the water bath must be higher than the level of H2O2 in the conical flask, to ensure even heating
- The conical flask can also be swirled gently to mix the contents and maintain an even temperature
Investigating the effect of substrate concentration on amylase activity using iodine
- In this investigation, the rate of substrate disappearance is used to compare rates of reaction under different conditions
- Amylase is a digestive enzyme that hydrolyses starch into maltose and glucose
- Amylase functions best at pH 7 and 37oC (all enzymes operate best under specific conditions)
- Amylase and starch are combined and this reaction mixture is then tested for starch at regular time intervals
- This can be done by taking samples from the reaction mixture at each time interval and adding each sample to some iodine in potassium iodide solution
- Starch forms a blue-black colour with this solution
- If no starch is present, the iodine solution remains yellow-brown
- In this way, the time taken for starch to be broken down can be measured
- The investigation can be repeated under different starch concentrations and the reaction rates can then be compared
- This experiment also can be adapted to measure the effects of altering pH, temperature or enzyme concentration
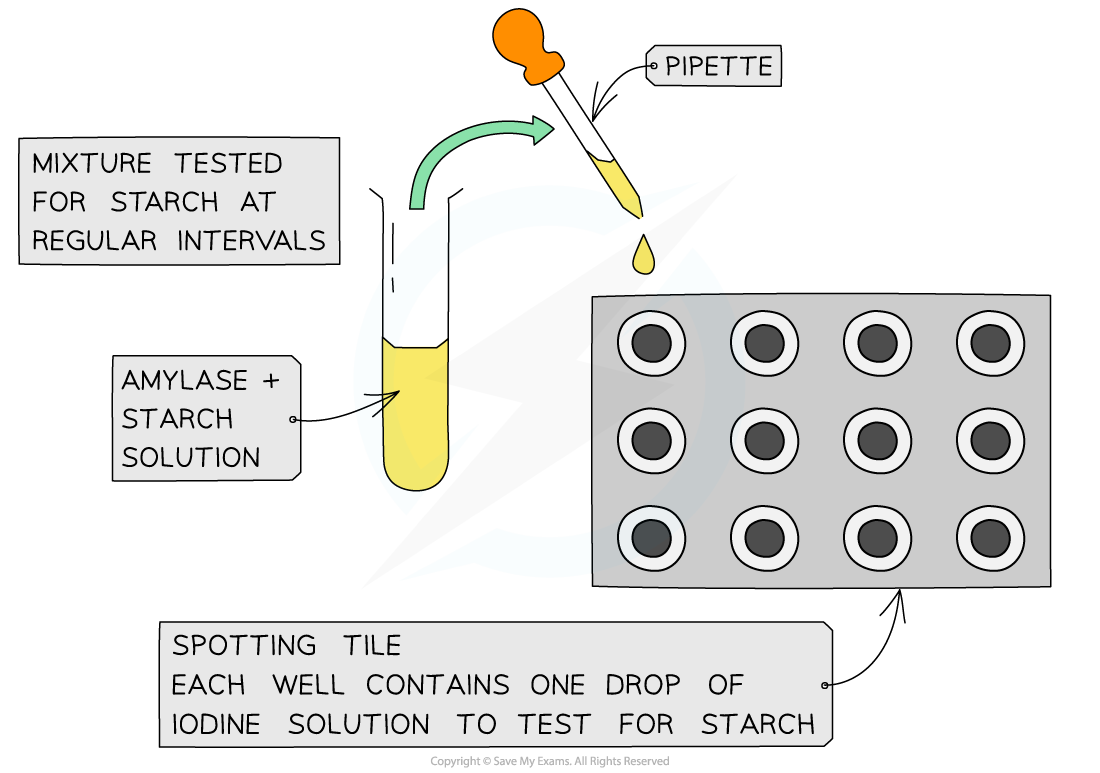
Experimental set-up for investigating the rate of disappearance of a substrate using amylase
Investigating the effect of starch concentration on amylase activity using colorimetry
- A colorimeter is able to measure light absorbance (how much light is absorbed) or light transmission (how much light passes through) a substance
- Colorimetry can be used in any enzyme-catalysed reaction that involves a colour change
- As the colour breaks down the transmission increases or light absorption decreases and this can be used to measure the rate of the reaction
- For example, a colorimeter can be used to follow the progress of a starch-amylase catalysed reaction as the amylase breaks the starch down into maltose
- This can be carried out as follows:
- Colorimeter calibration: this is an important step in a colorimetric investigation and in this case, a weak iodine solution can be used to calibrate the colorimeter as the endpoint (or 100% transmission)
- Preparation of a starch solution of known concentration (stock solution), from which a range of concentrations are made using serial dilutions (method outlined in diagram below)
- Following calibration and switching on the red filter (to maximise the percentage transmission or absorbance), the colorimeter is used to measure the percentage absorbance or percentage transmission values
- Sometimes a reagent or indicator is used to produce the colours detected by the colorimeter and sometimes the solutions themselves absorb light waves
- A calibration graph is then plotted of starch concentration (x-axis) vs percentage absorbance or percentage transmission (y-axis)
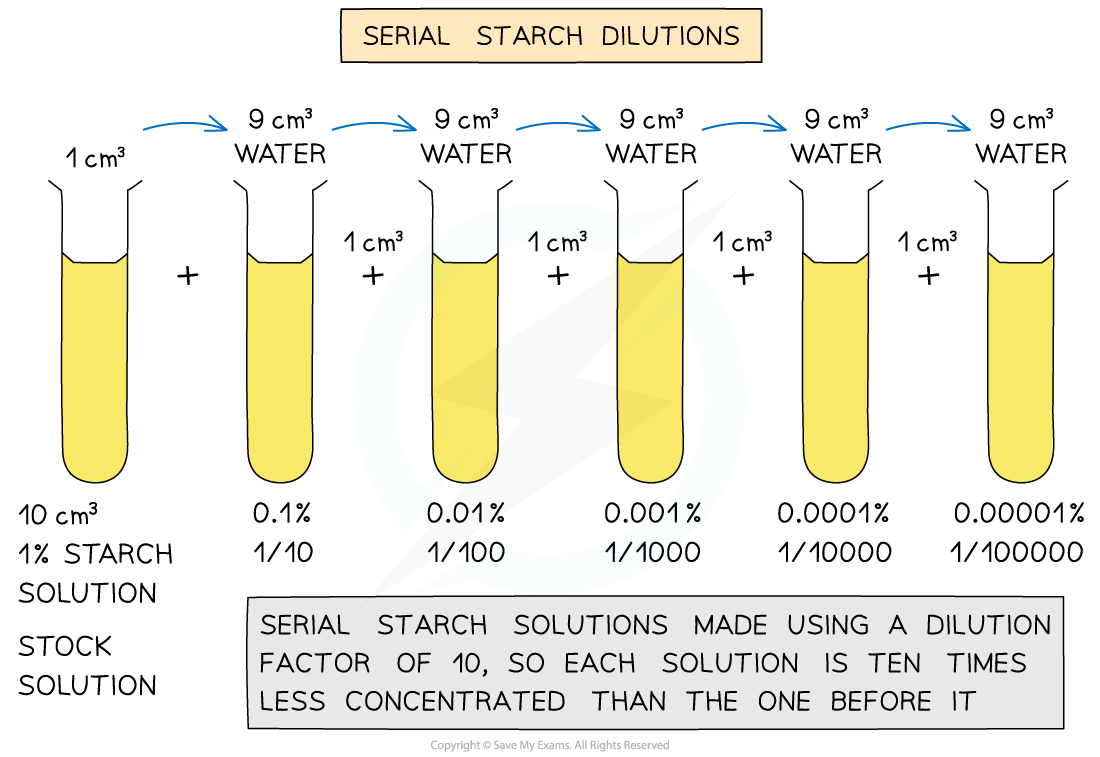
Serial dilution of starch to make a range of concentrations
NOS: Experimental design; accurate, quantitative measurements in enzyme experiments require replicates to ensure reliability
- Accurate measurements mean data that are close to the true value
- Quantitative measurements must be made
- A qualitative measurement might state that, "the enzyme worked at a faster rate at the higher temperature", whereas
- A quantitative measurement for the same experiment might state that, "the enzyme worked at a rate of 2.3 mmol product minute-1 at 40°C, versus 1.6 mmol product minute-1 at 25°C"
- Quantities, using numbers and appropriate units, are quoted in the experimental results
- Reliable data are generated from repeated experiments
- Anomalies can be identified and eliminated
- A reliable mean can be calculated from the data that remain
Exam Tip
RE-member: RE-peats bring RE-liability to experimental data.
